Feasibility of At-Home Serial Testing Using Over-the-Counter SARS-CoV-2 Tests With a Digital Smartphone App for Assistance: Longitudinal Cohort Study
- PMID: 36041004
- PMCID: PMC9580993
- DOI: 10.2196/35426
Feasibility of At-Home Serial Testing Using Over-the-Counter SARS-CoV-2 Tests With a Digital Smartphone App for Assistance: Longitudinal Cohort Study
Abstract
Background: The ongoing SARS-CoV-2 pandemic necessitates the development of accurate, rapid, and affordable diagnostics to help curb disease transmission, morbidity, and mortality. Rapid antigen tests are important tools for scaling up testing for SARS-CoV-2; however, little is known about individuals' use of rapid antigen tests at home and how to facilitate the user experience.
Objective: This study aimed to describe the feasibility and acceptability of serial self-testing with rapid antigen tests for SARS-CoV-2, including need for assistance and the reliability of self-interpretation.
Methods: A total of 206 adults in the United States with smartphones were enrolled in this single-arm feasibility study in February and March 2021. All participants were asked to self-test for COVID-19 at home using rapid antigen tests daily for 14 days and use a smartphone app for testing assistance and to report their results. The main outcomes were adherence to the testing schedule, the acceptability of testing and smartphone app experiences, and the reliability of participants versus study team's interpretation of test results. Descriptive statistics were used to report the acceptability, adherence, overall rating, and experience of using the at-home test and MyDataHelps app. The usability, acceptability, adherence, and quality of at-home testing were analyzed across different sociodemographic, age, and educational attainment groups.
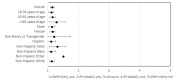
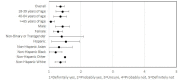
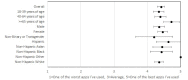
Results: Of the 206 enrolled participants, 189 (91.7%) and 159 (77.2%) completed testing and follow-up surveys, respectively. In total, 51.3% (97/189) of study participants were women, the average age was 40.7 years, 34.4% (65/189) were non-White, and 82% (155/189) had a bachelor's degree or higher. Most (n=133/206, 64.6%) participants showed high testing adherence, meaning they completed over 75% of the assigned tests. Participants' interpretations of test results demonstrated high agreement (2106/2130, 98.9%) with the study verified results, with a κ score of 0.29 (P<.001). Participants reported high satisfaction with self-testing and the smartphone app, with 98.7% (157/159) reporting that they would recommend the self-test and smartphone app to others. These results were consistent across age, race/ethnicity, and gender.
Conclusions: Participants' high adherence to the recommended testing schedule, significant reliability between participants and study staff's test interpretation, and the acceptability of the smartphone app and self-test indicate that self-tests for SARS-CoV-2 with a smartphone app for assistance and reporting is a highly feasible testing modality among a diverse population of adults in the United States.
Keywords: COVID-19; MyDataHelps smartphone app; SARS-CoV-2; digital health; mHealth; mobile health; pandemic; rapid tests; self test; serial self-testing.
©Carly Herbert, John Broach, William Heetderks, Felicia Qashu, Laura Gibson, Caitlin Pretz, Kelsey Woods, Vik Kheterpal, Thejas Suvarna, Christopher Nowak, Peter Lazar, Didem Ayturk, Bruce Barton, Chad Achenbach, Robert Murphy, David McManus, Apurv Soni. Originally published in JMIR Formative Research (https://formative.jmir.org), 18.10.2022.
Conflict of interest statement
Conflicts of Interest: CN and VK are employees of CareEvolution. DM has received personal fees and grants from Bristol Myers Squibb, Pfizer, Flexcon, and Fitbit; personal fees for editorial work from Heart Rhythm Society and from Avania for work on Data Safety Monitoring Boards; financial research support from Boehringer Ingelheim; and non-financial research support from Apple, Samsung, and Fitbit.
Figures


References
-
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. https://europepmc.org/abstract/MED/32087114 S1473-3099(20)30120-1 - DOI - PMC - PubMed
-
- Tromberg BJ, Schwetz TA, Pérez-Stable Eliseo J, Hodes RJ, Woychik RP, Bright RA, Fleurence RL, Collins FS. Rapid scaling up of COVID-19 diagnostic testing in the United States - the NIH RADx Initiative. N Engl J Med. 2020 Sep 10;383(11):1071–1077. doi: 10.1056/NEJMsr2022263. https://europepmc.org/abstract/MED/32706958 - DOI - PMC - PubMed
-
- Ricks S, Kendall EA, Dowdy DW, Sacks JA, Schumacher SG, Arinaminpathy N. Quantifying the potential value of antigen-detection rapid diagnostic tests for COVID-19: a modelling analysis. BMC Med. 2021 Mar 09;19(1):75. doi: 10.1186/s12916-021-01948-z. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-021-01948-z 10.1186/s12916-021-01948-z - DOI - DOI - PMC - PubMed
-
- Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Domen J, Dretzke J, Ferrante di Ruffano Lavinia, Harris I, Price M, Taylor-Phillips S, Hooft L, Leeflang M, McInnes M, Spijker R, Van den Bruel Ann, Cochrane COVID-19 Diagnostic Test Accuracy Group Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021 Mar 24;3:CD013705. doi: 10.1002/14651858.CD013705.pub2. https://europepmc.org/abstract/MED/33760236 - DOI - PMC - PubMed
-
- Nguyen N, Lane B, Lee IS, Gorman S, Wu Yumeng, Li A, Lu H, Elhadad N, Yin M, Meyers IK. A mixed methods study evaluating acceptability of a daily COVID-19 testing regimen with a mobile-app connected, at-home, rapid antigen test: implications for current and future pandemics. PLoS One. 2022 Aug 08;17(8):e0267766. doi: 10.1371/journal.pone.0267766. https://dx.plos.org/10.1371/journal.pone.0267766 PONE-D-22-10858 - DOI - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

