D-mannose for preventing and treating urinary tract infections
- PMID: 36041061
- PMCID: PMC9427198
- DOI: 10.1002/14651858.CD013608.pub2
D-mannose for preventing and treating urinary tract infections
Abstract
Background: Urinary tract infections (UTIs) are very common, affecting more than 7 million people worldwide. Whilst many people may only experience a single episode in their lifetime and are generally responsive to standard antibiotics, a significant proportion of adults and children (approximately 15% to 25%) are chronic symptomatic UTI sufferers. Certain population groups are at greater risk than others, such as immunosuppressed and people with chronic kidney disease. D-mannose is a sugar part of normal human metabolism found within most diets. The mechanism of action is to prevent bacterial adherence to the uroepithelial cells. The D-mannose-based inhibitors can block uropathogenic Escherichia coli adhesion and invasion of the uroepithelial cells. The bacteria are then understood to essentially be eliminated by urination. Early pilot studies on animals and humans have trialled concentrated forms of D-mannose (tablets or sachets) in doses ranging from 200 mg up to 2 to 3 g and found possible efficacy in reducing UTI symptoms or recurrence. Although the anti-adhesive effects of D-mannose have been well-established, only recently have we seen a small number of pilot studies and small clinical trials conducted.
Objectives: To assess the benefits and harms of D-mannose for preventing and treating UTIs in adults and children.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 22 February 2022 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included RCTs measuring and reporting the effect of D-mannose, in any combination and any formulation, to prevent or treat UTIs in adults and children, females and males, in any setting (including perioperative). Authors independently assessed the retrieved titles and abstracts and, where necessary, the full text to determine which satisfied the inclusion criteria.
Data collection and analysis: Data extraction was independently carried out by two authors using a standard data extraction form. Methodological quality of the included studies was assessed using the Cochrane risk of bias tool. Data entry was carried out by one author and cross-checked by another author. The certainty of the evidence was assessed using the GRADE approach.
Main results: We included seven RCTs (719 participants) in adult females and males who had either acute cystitis or a history of recurrent (at least two episodes in six months or three episodes in 12 months) UTIs (symptomatic or asymptomatic). Two were prevention studies, four were prevention and treatment studies (two perioperative and one in people with multiple sclerosis), and one was a treatment study. Time periods ranged from 15 days to six months. No two studies were comparable (by dose or treatments), and we could not undertake meta-analyses. Individual studies reported no clear evidence to determine whether D-mannose is more or less effective in preventing or treating UTIs. D-mannose (2 g) had uncertain effects on symptomatic and bacteriuria-confirmed UTIs when compared to no treatment (1 study, 205 participants; very low certainty evidence) and antibiotics (nitrofurantoin 50 mg) (1 study, 206 participants; very low certainty evidence). D-mannose, in combination with herbal supplements, had uncertain effects on symptomatic and bacteria-confirmed UTI and pain when compared to no treatment (1 study, 40 participants; very low certainty evidence). D-mannose 500 mg plus supplements (N-acetylcysteine and Morinda citrifolia fruit extract) had uncertain effects on symptomatic and bacteriuria-confirmed UTIs when compared to an antibiotic (prulifloxacin 400 mg) (1 study, 75 participants; very low certainty evidence). Adverse events were very few and poorly reported; none were serious (mostly diarrhoea and vaginal burning). Overall, the quality of the evidence is poor. Most studies were judged to have unclear or high risk of bias across most domains. Data was sparse and addressed very few outcomes. The GRADE evaluation was rated as very low certainty evidence due to very serious limitations in the study design or execution (high risk of bias across all studies) and sparse data (single study data and small sample sizes).
Authors' conclusions: There is currently little to no evidence to support or refute the use of D-mannose to prevent or treat UTIs in all populations. This review highlights the severe lack of high-quality RCTs testing the efficacy of D-mannose for UTIs in any population. Despite UTIs being one of the most common adult infections (affecting 50% of women at least once in their lifetime) and the growing global antimicrobial resistance, we found very few studies that adequately test this alternative treatment. Future research in this field requires, in the first instance, a single adequately powered RCT comparing D-mannose with placebo.
Trial registration: ClinicalTrials.gov NCT01808755 NCT03497598 NCT03996057 NCT03597152.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TC: no relevant interests were disclosed
CT: no relevant interests were disclosed
MH: no relevant interests were disclosed
ATP: no relevant interests were disclosed
AJ: no relevant interests were disclosed
GW: no relevant interests were disclosed
Figures
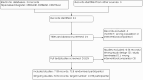








Comment in
-
Infection and Inflammation of the Genitourinary Tract.J Urol. 2023 Jul;210(1):202. doi: 10.1097/JU.0000000000003466. Epub 2023 Apr 24. J Urol. 2023. PMID: 37092709 No abstract available.
References
References to studies included in this review
De Leo 2017 {published data only}
-
- De Leo V, Cappelli V, Massaro MG, Tosti C, Morgante G. Evaluation of the effects of a natural dietary supplement with cranberry, Noxamicina ® and D-mannose in recurrent urinary infections in perimenopausal women. Minerva Ginecologica 2017;69(4):336-41. [MEDLINE: ] - PubMed
Kranjcec 2014 {published data only}
-
- Altarac S, Papes D. Use of D-mannose in prophylaxis of recurrent urinary tract infections (UTIs) in women. BJU International 2014;113(1):9-10. [MEDLINE: ] - PubMed
-
- Kranjcec B, Papes D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World Journal of Urology 2014;32(1):79-84. [MEDLINE: ] - PubMed
Kuzmenko 2019 {published data only}
-
- Kuzmenko AV, Kuzmenko VV, Gyaurgiev TA. Efficacy of combined antibacterial-prebiotic therapy in combination with D-mannose in women with uncomplicated lower urinary tract infection. Urologiia 2019;(6):38-43. [MEDLINE: ] - PubMed
Lopes de Carvalho 2012 {published data only}
-
- Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects [abstract no: 108]. Multiple Sclerosis 2012;18(5):S12-3. [EMBASE: 70762266]
Palleschi 2017 {published data only}
-
- Palleschi G, Carbone A, Zanello PP, Mele R, Leto A, Fuschi A, et al. Prospective study to compare antibiosis versus the association of N-acetylcysteine, D-mannose and Morinda citrifolia fruit extract in preventing urinary tract infections in patients submitted to urodynamic investigation. Archivio Italiano di Urologia, Andrologia 2017;89(1):45-50. [MEDLINE: ] - PubMed
Porru 2014 {published data only}
-
- Porru D, Parmigiani A, Tinelli C, Barletta D, Choussos D, Di Franco C, et al. Oral D-mannose in recurrent urinary tract infections in women: a pilot study. Journal of Clinical Urology 2014;7(3):208-13. [EMBASE: 372993100]
Russo 2019 {published data only}
-
- Russo E, Montt Guevara M, Giannini A, Mannella P, Palla G, Caretto M, et al. Cranberry, D-mannose and anti-inflammatory agents prevent lower urinary tract symptoms in women undergoing prolapse surgery. Climacteric 2019;23(2):201-5. [MEDLINE: ] - PubMed
References to studies excluded from this review
Domenici 2016 {published data only}
-
- Domenici L, Monti M, Bracchi C, Giorgini M, Colagiovanni V, Muzii L, et al. D-mannose: a promising support for acute urinary tract infections in women. A pilot study. European Review for Medical & Pharmacological Sciences 2016;20(13):2920-5. [MEDLINE: ] - PubMed
Genovese 2018 {published data only}13017713
-
- Genovese C, Davinelli S, Mangano K, Tempera G, Nicolosi D, Corsello S, et al. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. Journal of Chemotherapy 2018;30(2):107-14. [MEDLINE: ] - PubMed
NCT03497598 {published data only}
-
- Ryu G. Preventing recurrent urinary tract infections with a-d-mannose (PUTIM) [Preventing recurrent urinary tract infections with α-D-mannose: a prospective, randomized, double-blinded placebo-controlled trial]. www.clinicaltrials.gov/show/NCT03497598 (first received 13 April 2018).
NCT03996057 {published data only}
-
- Chu C. Methenamine in a non-antibiotic, multimodal approach to UTI prevention [The efficacy and effect of methenamine hippurate in a non-antibiotic, multimodal approach to UTI prevention]. www.clinicaltrials.gov/show/NCT03996057 (first received 24 June 2019).
Radulescu 2020 {published data only}
-
- Radulescu D, David C, Turcu FL, Spataru DM, Popescu P, Vacaroiu IA. Combination of cranberry extract and D-mannose - possible enhancer of uropathogen sensitivity to antibiotics in acute therapy of urinary tract infections: results of a pilot study. Experimental & Therapeutic Medicine 2020;20(4):3399-406. [MEDLINE: ] - PMC - PubMed
Salinas‐Casado 2018 {published data only}
-
- Salinas-Casado J, Mendez-Rubio S, Esteban-Fuertes M, Gomez-Rodriguez A, Virseda-Chamorro M, Lujan-Galan M, et al. Efficacy and safety of D-mannose (2 g), 24h prolonged release, associated with proanthocyanidin (PAC), versus isolate PAC, in the management of a series of women with recurrent urinary infections [Eficacia y tolerancia terapeutica de la D-manosa (2 g) de liberacion prolongada 24 horas (asociada a proantocianidinas), frente a proantocianidinas aisladas en el manejo de una serie de mujeres con infecciones urinarias recurrentes]. Archivos Espanoles de Urologia 2018;71(2):169-77. [MEDLINE: ] - PubMed
References to ongoing studies
ACTRN12616001619437 {published data only}
-
- Subramanian S. D-mannose for prophylaxis against urinary tract Infection in spinal cord injury [D-mannose for prophylaxis against urinary tract Infection in SCI: pilot randomised control study]. www.anzctr.org.au/ACTRN12616001619437.aspx (first received 23 November 2016).
ACTRN12619000183189 {published data only}
-
- Patton V. The effectiveness of D-Mannose in patients with high risk of recurrent urinary tract infections. www.anzctr.org.au/ACTRN12619000183189.aspx (first received 8 February 2019).
DRKS00013240 {published data only}
-
- Hellemann M. Effects of a food for special medical purposes containing d-mannose, birch extract, vitamin D and vitamin A for the dietary management of acute symptomatic uncomplicated urinary tract infections in females - a randomized, double-blind, placebo-controlled, parallel-design study. www.trialsearch.who.int/Trial2.aspx?TrialID=DRKS00013240 (first received 11 June 2017). [CENTRAL: CN-01890405]
MERIT 2021 {published data only}13283516
NCT03597152 {published data only}
-
- D'Anna R. Nutritional supplementation for recurrent urinary tract infections in women [Nutritional supplementation for extending time between recurrent urinary tract infections in women: a randomized double blind cross-over trial]. www.clinicaltrials.gov/show/NCT03597152 (first received 24 July 2018).
PROTON 2018 {published data only}
-
- Flach J. Winclove CLEAR for recurrent urinary tract infections in women [A randomized, placebo-controlled, pilot study to evaluate the effects winclove CLEAR in female recurrent urinary tract infection patients]. www.trialregister.nl/trial/6832 (first received 25 January 2018).
Additional references
Altarac 2014
-
- Altarac S, Papeš D. Use of d-mannose in prophylaxis of recurrent urinary tract infections (UTIs) in women. BJU International 2014;113(1):9-10. [MEDLINE: ] - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [MEDLINE: ] - PubMed
Foxman 2014
-
- Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious Disease Clinics of North America 2014;28(1):1-13. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hu 2016
-
- Hu X, Shi Y, Zhang P, Miao M, Zhang T, Jiang B. D-mannose: properties, production, and applications: an overview. Comprehensive Reviews in Food Science & Food Safety 2016;15(4):773-85. [MEDLINE: ] - PubMed
Kranjčec 2014
-
- Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World Journal of Urology 2014;32(1):79-84. [MEDLINE: ] - PubMed
Lenger 2020
Lopes De Carvalho 2012a
-
- Lopes De Carvalho L, Francavilla G, Motta R, Brichetto G. D-mannose, cranberry and Vitamin C are effective in preventing urinary tract infections in multiple sclerosis subjects (#108). Multiple Sclerosis 2012;18(5):S12-3. [EMBASE: 70762266]
Nicolle 2005
-
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults [Erratum in: Clin Infect Dis. 2005 May 15;40(10):1556]. Clinical Infectious Diseases 2005;40(5):643-54. [MEDLINE: ] - PubMed
Rowe 2014
Schunemann 2020a
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
Schunemann 2020b
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
US PSTF 2019
-
- US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for asymptomatic bacteriuria in adults: US Preventive Services Task Force recommendation statement. JAMA 2019;322(12):1188-94. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

