Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer
- PMID: 36041086
- PMCID: PMC10476837
- DOI: 10.1200/JCO.22.01643
Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer
Abstract
Purpose: Cisplatin-based combination chemotherapy remains the standard of care for locally advanced or metastatic urothelial cancer (la/mUC); however, toxicity is substantial, responses are rarely durable, and many patients with la/mUC are ineligible. Each enfortumab vedotin and pembrolizumab have shown a survival benefit versus chemotherapy in UC, are not restricted by cisplatin eligibility, and warrant investigation as a first-line (1L) combination therapy in patients ineligible for cisplatin.
Methods: In this ongoing phase Ib/II, multicenter, open-label study, 1L cisplatin-ineligible patients with la/mUC received enfortumab vedotin 1.25 mg/kg once daily on days 1 and 8 and pembrolizumab 200 mg (day 1) intravenously once daily in 3-week cycles. The primary end point was safety. Key secondary end points included confirmed objective response rate, duration of response (DOR), and overall survival (OS).
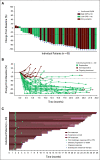
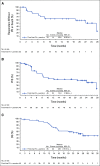
Results: Forty-five patients received enfortumab vedotin plus pembrolizumab. The most common treatment-related adverse events (TRAEs) were peripheral sensory neuropathy (55.6%), fatigue (51.1%), and alopecia (48.9%). Twenty-nine patients (64.4%) had grade 3 or higher TRAEs; the most common were increased lipase (17.8%), maculopapular rash (11.1%), and fatigue (11.1%). One death (2.2%) was classified as a TRAE. The confirmed objective response rate after a median of nine cycles was 73.3% with a complete response rate of 15.6%. The median DOR and median OS were 25.6 months and 26.1 months, respectively.
Conclusion: Enfortumab vedotin plus pembrolizumab showed a manageable safety profile. Most patients experienced tumor shrinkage. The median DOR and median OS exceeding 2 years in a cisplatin-ineligible patient population make this a promising combination currently under investigation in a phase III study (ClinicalTrials.gov identifier: NCT04223856).
Trial registration: ClinicalTrials.gov NCT03288545 NCT04223856.
Conflict of interest statement
Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Dash A, Galsky MD, Vickers AJ, et al. : Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 107:506-513, 2006 - PubMed
-
- Galsky MD, Hahn NM, Rosenberg J, et al. : Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol 29:2432-2438, 2011 - PubMed
-
- Sternberg CN, de Mulder P, Schornagel JH, et al. : Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42:50-54, 2006 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

