Cathepsin D Drives the Formation of Hybrid Insulin Peptides Relevant to the Pathogenesis of Type 1 Diabetes
- PMID: 36041196
- PMCID: PMC9750942
- DOI: 10.2337/db22-0303
Cathepsin D Drives the Formation of Hybrid Insulin Peptides Relevant to the Pathogenesis of Type 1 Diabetes
Abstract
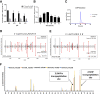
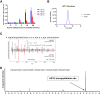
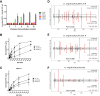
Hybrid insulin peptides (HIPs) form in pancreatic β-cells through the formation of peptide bonds between proinsulin fragments and other peptides. HIPs have been identified in pancreatic islets by mass spectrometry and are targeted by CD4 T cells in patients with type 1 diabetes (T1D) as well as by pathogenic CD4 T-cell clones in nonobese diabetic (NOD) mice. The mechanism of HIP formation is currently poorly understood; however, it is well established that proteases can drive the formation of new peptide bonds in a side reaction during peptide bond hydrolysis. Here, we used a proteomic strategy on enriched insulin granules and identified cathepsin D (CatD) as the primary protease driving the specific formation of HIPs targeted by disease-relevant CD4 T cells in T1D. We also established that NOD islets deficient in cathepsin L (CatL), another protease implicated in the formation of disease-relevant HIPs, contain elevated levels of HIPs, indicating a role for CatL in the proteolytic degradation of HIPs. In summary, our data suggest that CatD may be a therapeutic target in efforts to prevent or slow the autoimmune destruction of β-cells mediated by HIP-reactive CD4 T cells in T1D.
© 2022 by the American Diabetes Association.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

