Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension
- PMID: 36041750
- PMCID: PMC9816418
- DOI: 10.1183/13993003.01347-2022
Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension
Abstract
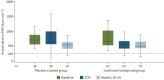
Background: In participants with pulmonary arterial hypertension, 24 weeks of sotatercept resulted in a significantly greater reduction from baseline in pulmonary vascular resistance than placebo. This report characterises the longer-term safety and efficacy of sotatercept in the PULSAR open-label extension. We report cumulative safety, and efficacy at months 18-24, for all participants treated with sotatercept.
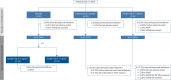
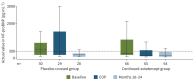
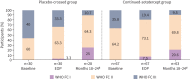
Methods: PULSAR was a phase 2, randomised, double-blind, placebo-controlled study followed by an open-label extension, which evaluated sotatercept on top of background pulmonary arterial hypertension therapy in adults. Participants originally randomised to placebo were re-randomised 1:1 to sotatercept 0.3 or 0.7 mg·kg-1 (placebo-crossed group); those initially randomised to sotatercept continued the same sotatercept dose (continued-sotatercept group). Safety was evaluated in all participants who received ≥1 dose of sotatercept. The primary efficacy endpoint was change from baseline to months 18-24 in pulmonary vascular resistance. Secondary endpoints included 6-min walk distance and functional class. Two prespecified analyses, placebo-crossed and delayed-start, evaluated efficacy irrespective of dose.
Results: Of 106 participants enrolled in the PULSAR study, 97 continued into the extension period. Serious treatment-emergent adverse events were reported in 32 (30.8%) participants; 10 (9.6%) reported treatment-emergent adverse events leading to study discontinuation. Three (2.9%) participants died, none considered related to study drug. The placebo-crossed group demonstrated significant improvement across primary and secondary endpoints and clinical efficacy was maintained in the continued-sotatercept group.
Conclusion: These results support the longer-term safety and durability of clinical benefit of sotatercept for pulmonary arterial hypertension.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: M. Humbert is a consultant and an advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aerovate, Altavant, AOP Orphan, Bayer, Ferrer, Janssen, Merck & Co., Inc., Rahway, NJ, USA, MorphogenIX and United Therapeutics, and has received research grants for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, and Merck & Co., Inc., Rahway, NJ, USA. V. McLaughlin is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aerovate, Altavant, Bayer, Caremark LLC, CiVi Bioharma Inc., Corvista, Gossamer Bio, Janssen, Merck & Co., Inc., Rahway, NJ, USA, and United Therapeutics, has received grant(s) from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, Sonovie, United Therapeutics, is involved in CME programs for Impact PH and PHA, and on the board of directors for CiVi Biopharma Inc., and for Clene. J.S.R. Gibbs is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Aerovate, Bayer, Complexa, Janssen, Merck & Co., Inc., Rahway, NJ, USA, MSD, Pfizer and United Therapeutics. M. Gomberg-Maitland is a consultant for Altavant, Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, and Entelligence Young for Janssen, is part of the advisory committee for United Therapeutics and the data safety monitoring board for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, has received grant(s) and/or reports GW with funds from Altavant, Bayer and United Therapeutics. M.M. Hoeper is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer. I.R. Preston is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Pfizer, Respira and United Therapeutics, a steering committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has received grant(s) from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Complexa, Liquidia, PhaseBio, Tenax and United Therapeutics. R. Souza is an advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A.B. Waxman is steering committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Altavant, Gossamer Bio, United Therapeutics, an investigator and/or principal investigator for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aria-CV and United Therapeutics, and has received grant(s) from Acceleron, a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. H-A. Ghofrani is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Gossamer Bio, Jansson, MorphogenIX, MSD and Pfizer. P. Escribano Subias has received consulting fees from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, MSD and Gossamer Bio, has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Janssen, MSD, Ferrer, Bayer and AOT, has received support for attending meetings from Janssen and MSD, has received equipment, materials, drugs, medical writing or other services from Janssen, and has participated on data safety monitoring or advisory boards for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway NJ, USA, Janssen, MSD and Gossamer Bio. J. Feldman reports consulting fees from Merck, Aerovate, Altavant, United Therapeutics, Liquidia, Corsair and Janssen. G. Meyer has received payment or honoraria for lectures, presentations, and speakers’ bureaus from Bayer and Janssen and has participated on advisory boards for Bayer and Janssen. D. Montani has received grant(s) from and is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Chesi, GlaxoSmithKline, MSD and Pfizer. K.M. Olsson is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer. S. Manimaran and J. de Oliveira Pena are employees of Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. D.B. Badesch is a consultant for Pfizer, is a consultant and advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Altavant, Arena, Bayer, Liquidia, Merck & Co., Inc., Rahway, NJ, USA, United Therapeutics, has received research grant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Altavant, Arena, Belleraphon, Janssen, Liquidia, Merck & Co., Inc., Rahway, NJ, USA, and United Therapeutics, sits on the data safety monitoring board for United Therapeutics, and is a long-term holder of common stock for Johnson and Johnson.
Figures

Comment in
-
Sotatercept for pulmonary arterial hypertension: something old and something new.Eur Respir J. 2023 Jan 6;61(1):2201972. doi: 10.1183/13993003.01972-2022. Print 2023 Jan. Eur Respir J. 2023. PMID: 36609525 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
