Nintedanib in children and adolescents with fibrosing interstitial lung diseases
- PMID: 36041751
- PMCID: PMC9892863
- DOI: 10.1183/13993003.01512-2022
Nintedanib in children and adolescents with fibrosing interstitial lung diseases
Erratum in
-
"Nintedanib in children and adolescents with fibrosing interstitial lung diseases." R. Deterding, L.R. Young, E.M. DeBoer, D. Warburton, S. Cunningham, N. Schwerk, K.R. Flaherty, K.K. Brown, M. Dumistracel, E. Erhardt, J. Bertulis, M. Gahlemann, S. Stowasser and M. Griese for the InPedILD trial investigators. Eur Respir J 2023; 61: 2201512.Eur Respir J. 2023 May 25;61(5):2251512. doi: 10.1183/13993003.51512-2022. Print 2023 May. Eur Respir J. 2023. PMID: 37230503 No abstract available.
Abstract
Background: Childhood interstitial lung disease (ILD) comprises a spectrum of rare ILDs affecting infants, children and adolescents. Nintedanib is a licensed treatment for pulmonary fibrosis in adults. The primary objectives of the InPedILD trial were to determine the dose-exposure and safety of nintedanib in children and adolescents with fibrosing ILD.
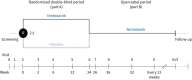
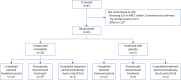
Methods: Patients aged 6-17 years with fibrosing ILD on high-resolution computed tomography and clinically significant disease were randomised 2:1 to receive nintedanib or placebo for 24 weeks and then open-label nintedanib. Dosing was based on weight-dependent allometric scaling. Co-primary end-points were the area under the plasma concentration-time curve at steady state (AUCτ,ss) at weeks 2 and 26 and the proportion of patients with treatment-emergent adverse events at week 24.
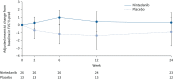
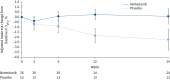
Results: 26 patients received nintedanib and 13 patients received placebo. The geometric mean (geometric coefficient of variation) AUCτ,ss for nintedanib was 175 µg·h·L-1 (85.1%) in patients aged 6-11 years and 160 µg·h·L-1 (82.7%) in patients aged 12-17 years. In the double-blind period, adverse events were reported in 84.6% of patients in each treatment group. Two patients discontinued nintedanib due to adverse events. Diarrhoea was reported in 38.5% and 15.4% of the nintedanib and placebo groups, respectively. Adjusted mean±se changes in percentage predicted forced vital capacity at week 24 were 0.3±1.3% in the nintedanib group and -0.9±1.8% in the placebo group.
Conclusions: In children and adolescents with fibrosing ILD, a weight-based dosing regimen resulted in exposure to nintedanib similar to adults and an acceptable safety profile. These data provide a scientific basis for the use of nintedanib in this patient population.
Trial registration: ClinicalTrials.gov NCT04093024.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: R. Deterding reports consultancy fees, fees for participation on the steering committee of the InPedILD trial and support for travel from Boehringer Ingelheim (BI); and consultancy fees from Roche. L.R. Young reports grants paid to her institution from the National Institutes of Health and Orphan Disease Center at the University of Pennsylvania; royalties from UpToDate; consultancy fees and fees for participation on the steering committee of the InPedILD trial from BI; consultancy fees from Roche and Sanofi; and honoraria from NYU Langone Health. E.M. DeBoer reports fees from BI for participation on the steering committee of the InPedILD trial. S. Cunningham reports fees paid to his institution by BI for his participation on the steering committee of the InPedILD trial. D. Warburton reports a grant re: the RECOVER (COVID-19) initiative from the National Heart, Lung, and Blood Institute (NHLBI) and fees from BI for participation on the steering committee of the InPedILD trial. N. Schwerk reports consultancy fees from Novartis; payment for lectures from AbbVie, Allergopharma, Chiesi, Infectopharm, Novartis, Pfizer and Sanofi; fees for participation in data safety monitoring boards or advisory boards for BI and Novartis; and fees from BI for participation on the steering committee of the InPedILD trial; he is a member of the German Society for Pediatric Pneumology, European Respiratory Society, Children's Interstitial Lung Disease Clinical Research Collaboration (chILD-EU), European Cooperation in Science and Technology (COST) network for translational research in children's and adult ILD (ENTeR-chILD), and Secretary of Kinderlungenregister. K.R. Flaherty reports grants paid to his institution from BI; royalties from UpToDate; and consultancy fees from Arrowhead, AstraZeneca, Bellerophon, BI, CSL Behring, Daewoong, DevPro, Dispersol, FibroGen, Horizon, Immunet, Lupin, NeRRe Therapeutics, Pliant, Polarean, Pure Health, PureTech, Respivant, Roche/Genentech, Shionogi, Sun Pharmaceuticals, Trevi and United Therapeutics; he is a steering committee chair for the Pulmonary Fibrosis Foundation and reports fees from BI for participation on the steering committee of the InPedILD trial. K.K. Brown reports grants from NHLBI; consultancy fees, speaker fees, support for travel and/or has served as an advisor or on a data monitoring committee for AbbVie, Biogen, Blade Therapeutics, BI, Bristol Myers Squibb, CSL Behring, DevPro Biopharma, Dispersol, Eleven P15, Galapagos, Galecto, Huitai Biomedcine, Humanetics, the Open Source Imaging Consortium (OSIC), Pliant, Redx Pharma, Sanofi, Third Pole and Translate Bio; and fees from BI for participation on the steering committee of the InPedILD trial; he holds leadership or fiduciary roles with the Fleischner Society and OSIC. M. Dumistracel, E. Erhardt, J. Bertulis, M. Gahlemann and S. Stowasser are employees of BI. M. Griese reports grants from BI for analysis of the chILD-EU register; has received fees from BI for membership of the InPedILD steering committee, an advisory board and an adjudication board, and for a web-based series.
Figures
Comment in
-
Nintedanib in chILD: a small step, yes… but at least a step forward in a marathon!Eur Respir J. 2023 Feb 2;61(2):2201797. doi: 10.1183/13993003.01797-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36731900 No abstract available.
Similar articles
-
Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial.Lancet Respir Med. 2020 May;8(5):453-460. doi: 10.1016/S2213-2600(20)30036-9. Epub 2020 Mar 5. Lancet Respir Med. 2020. PMID: 32145830 Clinical Trial.
-
Estimating the effect of nintedanib on forced vital capacity in children and adolescents with fibrosing interstitial lung disease using a Bayesian dynamic borrowing approach.Pediatr Pulmonol. 2024 Apr;59(4):1038-1046. doi: 10.1002/ppul.26882. Epub 2024 Jan 30. Pediatr Pulmonol. 2024. PMID: 38289091
-
Nintedanib: A Review in Fibrotic Interstitial Lung Diseases.Drugs. 2021 Apr;81(5):575-586. doi: 10.1007/s40265-021-01487-0. Epub 2021 Mar 25. Drugs. 2021. PMID: 33765296 Free PMC article. Review.
-
Study design of a randomised, placebo-controlled trial of nintedanib in children and adolescents with fibrosing interstitial lung disease.ERJ Open Res. 2021 Jun 21;7(2):00805-2020. doi: 10.1183/23120541.00805-2020. eCollection 2021 Apr. ERJ Open Res. 2021. PMID: 34164554 Free PMC article.
-
Progressive fibrosing interstitial lung diseases: A new concept and indication of nintedanib.Mod Rheumatol. 2021 Jan;31(1):13-19. doi: 10.1080/14397595.2020.1826665. Mod Rheumatol. 2021. PMID: 32964766 Review.
Cited by
-
The US national registry for childhood interstitial and diffuse lung disease: Report of study design and initial enrollment cohort.Pediatr Pulmonol. 2024 Sep;59(9):2236-2246. doi: 10.1002/ppul.26568. Epub 2023 Jul 4. Pediatr Pulmonol. 2024. PMID: 37401889
-
Clinical and research innovations in childhood interstitial lung disease (chILD).Pediatr Pulmonol. 2024 Sep;59(9):2233-2235. doi: 10.1002/ppul.27025. Epub 2024 Apr 23. Pediatr Pulmonol. 2024. PMID: 38651871 No abstract available.
-
A Crossroads for Corticosteroid Therapy in Pediatric Interstitial and Rare Lung Diseases.Ann Am Thorac Soc. 2025 May;22(5):660-661. doi: 10.1513/AnnalsATS.202411-1138VP. Ann Am Thorac Soc. 2025. PMID: 39918993 Free PMC article. No abstract available.
-
Innovations in Childhood Interstitial and Diffuse Lung Disease.Clin Chest Med. 2024 Sep;45(3):695-715. doi: 10.1016/j.ccm.2024.04.002. Clin Chest Med. 2024. PMID: 39069332 Free PMC article. Review.
-
An update on diagnosis and treatments of childhood interstitial lung diseases.Breathe (Sheff). 2025 May 13;21(2):250004. doi: 10.1183/20734735.0004-2025. eCollection 2025 Apr. Breathe (Sheff). 2025. PMID: 40365093 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
