AIMP1 promotes multiple myeloma malignancy through interacting with ANP32A to mediate histone H3 acetylation
- PMID: 36042007
- PMCID: PMC9648396
- DOI: 10.1002/cac2.12356
AIMP1 promotes multiple myeloma malignancy through interacting with ANP32A to mediate histone H3 acetylation
Abstract
Background: Multiple myeloma (MM) is the second most common hematological malignancy. An overwhelming majority of patients with MM progress to serious osteolytic bone disease. Aminoacyl-tRNA synthetase-interacting multifunctional protein 1 (AIMP1) participates in several steps during cancer development and osteoclast differentiation. This study aimed to explore its role in MM.
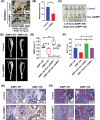
Methods: The gene expression profiling cohorts of MM were applied to determine the expression of AIMP1 and its association with MM patient prognosis. Enzyme-linked immunosorbent assay, immunohistochemistry, and Western blotting were used to detect AIMP1 expression. Protein chip analysis, RNA-sequencing, and chromatin immunoprecipitation and next-generation sequencing were employed to screen the interacting proteins and key downstream targets of AIMP1. The impact of AIMP1 on cellular proliferation was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in vitro and a xenograft model in vivo. Bone lesions were evaluated using tartrate-resistant acid phosphatase staining in vitro. A NOD/SCID-TIBIA mouse model was used to evaluate the effect of siAIMP1-loaded exosomes on bone lesion formation in vivo.
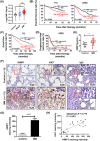
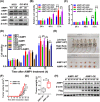
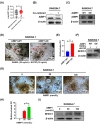
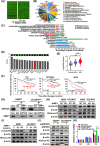
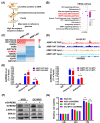
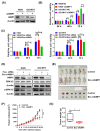
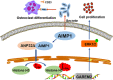
Results: AIMP1 expression was increased in MM patients and strongly associated with unfavorable outcomes. Increased AIMP1 expression promoted MM cell proliferation in vitro and in vivo via activation of the mitogen-activated protein kinase (MAPK) signaling pathway. Protein chip assays and subsequent experiments revealed that AIMP1 interacted with acidic leucine-rich nuclear phosphoprotein 32 family member A (ANP32A) to regulate histone H3 acetylation. In addition, AIMP1 increased histone H3 acetylation enrichment function of GRB2-associated and regulator of MAPK protein 2 (GAREM2) to increase the phosphorylation of extracellular-regulated kinase 1/2 (p-ERK1/2). Furthermore, AIMP1 promoted osteoclast differentiation by activating nuclear factor of activated T cells c1 (NFATc1) in vitro. In contrast, exosome-coated small interfering RNA of AIMP1 effectively suppressed MM progression and osteoclast differentiation in vitro and in vivo.
Conclusions: Our data demonstrate that AIMP1 is a novel regulator of histone H3 acetylation interacting with ANP32A in MM, which accelerates MM malignancy via activation of the MAPK signaling pathway.
Keywords: AIMP1; ANP32A; MAPK signaling; histone H3 acetylation; multiple myeloma; osteoclast differentiation; osteolytic lesions.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

References
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. - PubMed
-
- Rome S, Noonan K, Bertolotti P, Tariman JD, Miceli T. Bone Health, Pain, and Mobility: Evidence‐Based Recommendations for Patients With Multiple Myeloma. Clin J Oncol Nurs. 2017;21(5 Suppl):47–59. - PubMed
-
- Westhrin M, Kovcic V, Zhang Z, Moen SH, Nedal TMV, Bondt A, et al. Monoclonal immunoglobulins promote bone loss in multiple myeloma. Blood. 2020;136(23):2656–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

