Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy
- PMID: 36042210
- PMCID: PMC9428140
- DOI: 10.1038/s41467-022-32054-0
Organic phosphorescent nanoscintillator for low-dose X-ray-induced photodynamic therapy
Abstract
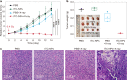
X-ray-induced photodynamic therapy utilizes penetrating X-rays to activate reactive oxygen species in deep tissues for cancer treatment, which combines the advantages of photodynamic therapy and radiotherapy. Conventional therapy usually requires heavy-metal-containing inorganic scintillators and organic photosensitizers to generate singlet oxygen. Here, we report a more convenient strategy for X-ray-induced photodynamic therapy based on a class of organic phosphorescence nanoscintillators, that act in a dual capacity as scintillators and photosensitizers. The resulting low dose of 0.4 Gy and negligible adverse effects demonstrate the great potential for the treatment of deep tumours. These findings provide an optional route that leverages the optical properties of purely organic scintillators for deep-tissue photodynamic therapy. Furthermore, these organic nanoscintillators offer an opportunity to expand applications in the fields of biomaterials and nanobiotechnology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

