A Pilot randomized trial to examine effects of a hybrid closed-loop insulin delivery system on neurodevelopmental and cognitive outcomes in adolescents with type 1 diabetes
- PMID: 36042217
- PMCID: PMC9427757
- DOI: 10.1038/s41467-022-32289-x
A Pilot randomized trial to examine effects of a hybrid closed-loop insulin delivery system on neurodevelopmental and cognitive outcomes in adolescents with type 1 diabetes
Abstract
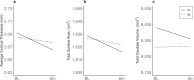
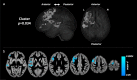
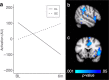
Type 1 diabetes (T1D) is associated with lower scores on tests of cognitive and neuropsychological function and alterations in brain structure and function in children. This proof-of-concept pilot study (ClinicalTrials.gov Identifier NCT03428932) examined whether MRI-derived indices of brain development and function and standardized IQ scores in adolescents with T1D could be improved with better diabetes control using a hybrid closed-loop insulin delivery system. Eligibility criteria for participation in the study included age between 14 and 17 years and a diagnosis of T1D before 8 years of age. Randomization to either a hybrid closed-loop or standard diabetes care group was performed after pre-qualification, consent, enrollment, and collection of medical background information. Of 46 participants assessed for eligibility, 44 met criteria and were randomized. Two randomized participants failed to complete baseline assessments and were excluded from final analyses. Participant data were collected across five academic medical centers in the United States. Research staff scoring the cognitive assessments as well as those processing imaging data were blinded to group status though participants and their families were not. Forty-two adolescents, 21 per group, underwent cognitive assessment and multi-modal brain imaging before and after the six month study duration. HbA1c and sensor glucose downloads were obtained quarterly. Primary outcomes included metrics of gray matter (total and regional volumes, cortical surface area and thickness), white matter volume, and fractional anisotropy. Estimated power to detect the predicted treatment effect was 0.83 with two-tailed, α = 0.05. Adolescents in the hybrid closed-loop group showed significantly greater improvement in several primary outcomes indicative of neurotypical development during adolescence compared to the standard care group including cortical surface area, regional gray volumes, and fractional anisotropy. The two groups were not significantly different on total gray and white matter volumes or cortical thickness. The hybrid closed loop group also showed higher Perceptual Reasoning Index IQ scores and functional brain activity more indicative of neurotypical development relative to the standard care group (both secondary outcomes). No adverse effects associated with study participation were observed. These results suggest that alterations to the developing brain in T1D might be preventable or reversible with rigorous glucose control. Long term research in this area is needed.
© 2022. The Author(s).
Conflict of interest statement
N.M. had institutional device supply agreements from Medtronic for CGM and LifeScan for test strips for the study, research support from Novo Nordisk. B.B. had consultant agreements with Medtronic Diabetes, Novo Nordisk, Dexcom, ConvaTec, Lilly, and Tolerion; has provided expert testimony for Dexcom; and has research grants with Insulet, Tandem, Medtronic, and Beta Bionics. E.T. had institutional research grants with AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Grifols Therapeutics, Takeda, and Amgen. S.A.W. had consultant agreements with Zealand; institutional grant support from Abbott and Medtronic; and speaker honoraria from Abbott, Dexcom, and Insulet. L.A.F. had a device supply agreement with Dexcom. A.M.A. had a device supply agreement with Dexcom. M.T. had a data safety monitoring board agreement with Daiichi Sankyo. W.T. had consultant agreements with AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi and data safety monitoring board agreements with Eisai, MannKind, and Tolerion. K.E. had a consultant agreement with PicoLife Technology. J.L. received consulting fees, paid to his institution, from Animas Corporation, Bigfoot Biomedical, Tandem Diabetes Care, and Eli Lilly and Company. No other potential competing interests relevant to this article were reported.
Figures



References
-
- Bober E, Buyukgebiz A. Hypoglycemia and its effects on the brain in children with type 1 diabetes mellitus. Pediatr. Endocrinol. Rev. 2005;2:378–382. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

