Structure and dynamics of Toll immunoreceptor activation in the mosquito Aedes aegypti
- PMID: 36042238
- PMCID: PMC9427763
- DOI: 10.1038/s41467-022-32690-6
Structure and dynamics of Toll immunoreceptor activation in the mosquito Aedes aegypti
Abstract
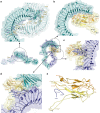
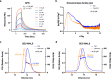
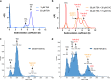
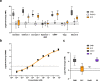
Aedes aegypti has evolved to become an efficient vector for arboviruses but the mechanisms of host-pathogen tolerance are unknown. Immunoreceptor Toll and its ligand Spaetzle have undergone duplication which may allow neofunctionalization and adaptation. Here we present cryo-EM structures and biophysical characterisation of low affinity Toll5A complexes that display transient but specific interactions with Spaetzle1C, forming asymmetric complexes, with only one ligand clearly resolved. Loop structures of Spaetzle1C and Toll5A intercalate, temporarily bridging the receptor C-termini to promote signalling. By contrast unbound receptors form head-to-head homodimers that keep the juxtamembrane regions far apart in an inactive conformation. Interestingly the transcriptional signature of Spaetzle1C differs from other Spaetzle cytokines and controls genes involved in innate immunity, metabolism and tissue regeneration. Taken together our results explain how upregulation of Spaetzle1C in the midgut and Toll5A in the salivary gland shape the concomitant immune response.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hoffmann, J. A. The immune response of Drosophila. Nature426, 33–38 (2003). - PubMed
-
- Hoffmann A, Neumann P, Schierhorn A, Stubbs MT. Crystallization of Spatzle, a cystine-knot protein involved in embryonic development and innate immunity in Drosophila melanogaster. Acta Crystallogr. Sect. F.-Struct. Biol. Cryst. Commun. 2008;64:707–710. doi: 10.1107/S1744309108018812. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

