Tackling recalcitrant Pseudomonas aeruginosa infections in critical illness via anti-virulence monotherapy
- PMID: 36042245
- PMCID: PMC9428149
- DOI: 10.1038/s41467-022-32833-9
Tackling recalcitrant Pseudomonas aeruginosa infections in critical illness via anti-virulence monotherapy
Abstract
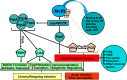
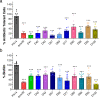
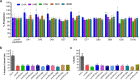
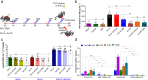
Intestinal barrier derangement allows intestinal bacteria and their products to translocate to the systemic circulation. Pseudomonas aeruginosa (PA) superimposed infection in critically ill patients increases gut permeability and leads to gut-driven sepsis. PA infections are challenging due to multi-drug resistance (MDR), biofilms, and/or antibiotic tolerance. Inhibition of the quorum-sensing transcriptional regulator MvfR(PqsR) is a desirable anti-PA anti-virulence strategy as MvfR controls multiple acute and chronic virulence functions. Here we show that MvfR promotes intestinal permeability and report potent anti-MvfR compounds, the N-Aryl Malonamides (NAMs), resulting from extensive structure-activity-relationship studies and thorough assessment of the inhibition of MvfR-controlled virulence functions. This class of anti-virulence non-native ligand-based agents has a half-maximal inhibitory concentration in the nanomolar range and strong target engagement. Using a NAM lead in monotherapy protects murine intestinal barrier function, abolishes MvfR-regulated small molecules, ameliorates bacterial dissemination, and lowers inflammatory cytokines. This study demonstrates the importance of MvfR in PA-driven intestinal permeability. It underscores the utility of anti-MvfR agents in maintaining gut mucosal integrity, which should be part of any successful strategy to prevent/treat PA infections and associated gut-derived sepsis in critical illness settings. NAMs provide for the development of crucial preventive/therapeutic monotherapy options against untreatable MDR PA infections.
© 2022. The Author(s).
Conflict of interest statement
L.G.R. has a financial interest in Spero Therapeutics, a company developing therapies to treat bacterial infections. L.G.R.’s financial interests are reviewed and managed by Massachusetts General Hospital and Mass General Brigham Integrated Health Care System in accordance with their conflict-of-interest policies. No funding was received from Spero Therapeutics and had no role in study design, data collection, analysis, interpretation, or the decision to submit the work for publication. The remaining authors declare no competing interests. Patent: Broad Sepctrum anti-virulence anti-persistence compounds. Inventors: Laurence Rahme, Francois Lepine, Damien Maura, Carmella Napolitano, Antonio Felice, Michele Negri, Stefano Fontana, Daniele Andreotti. Institution: Massachusetts General Hospital. Publication number: 20210130306. Filed October 19, 2018. Publication date: May 6, 2021. All compounds reported in this publication are included in the aforementioned patent.
Figures


References
-
- CDC. (ed CDC) (2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

