Behaviorally relevant decision coding in primary somatosensory cortex neurons
- PMID: 36042310
- PMCID: PMC7613627
- DOI: 10.1038/s41593-022-01151-0
Behaviorally relevant decision coding in primary somatosensory cortex neurons
Erratum in
-
Author Correction: Behaviorally relevant decision coding in primary somatosensory cortex neurons.Nat Neurosci. 2023 Jan;26(1):173. doi: 10.1038/s41593-022-01234-y. Nat Neurosci. 2023. PMID: 36414687 No abstract available.
Abstract
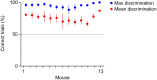
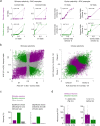
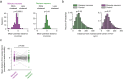
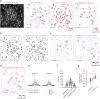
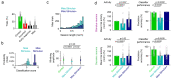
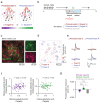
Primary sensory cortex is thought to process incoming sensory information, while decision variables important for driving behavior are assumed to arise downstream in the processing hierarchy. Here, we used population two-photon calcium imaging and targeted two-photon optogenetic stimulation of neurons in layer 2/3 of mouse primary somatosensory cortex (S1) during a texture discrimination task to test for the presence of decision signals and probe their behavioral relevance. Small but distinct populations of neurons carried information about the stimulus irrespective of the behavioral outcome (stimulus neurons), or about the choice irrespective of the presented stimulus (decision neurons). Decision neurons show categorical coding that develops during learning, and lack a conclusive decision signal in Miss trials. All-optical photostimulation of decision neurons during behavior improves behavioral performance, establishing a causal role in driving behavior. The fact that stimulus and decision neurons are intermingled challenges the idea of S1 as a purely sensory area, and causal perturbation suggests a direct involvement of S1 decision neurons in the decision-making process.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
A latent pool of neurons silenced by sensory-evoked inhibition can be recruited to enhance perception.Neuron. 2024 Jul 17;112(14):2386-2403.e6. doi: 10.1016/j.neuron.2024.04.015. Epub 2024 May 9. Neuron. 2024. PMID: 38729150 Free PMC article.
-
Sequence Learning Induces Selectivity to Multiple Task Parameters in Mouse Somatosensory Cortex.Curr Biol. 2021 Feb 8;31(3):473-485.e5. doi: 10.1016/j.cub.2020.10.059. Epub 2020 Nov 12. Curr Biol. 2021. PMID: 33186553 Free PMC article.
-
Behaviour-dependent recruitment of long-range projection neurons in somatosensory cortex.Nature. 2013 Jul 18;499(7458):336-40. doi: 10.1038/nature12236. Epub 2013 Jun 23. Nature. 2013. PMID: 23792559
-
Multielectrode Recordings in the Somatosensory System.In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 6. In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 6. PMID: 21204443 Free Books & Documents. Review.
-
Neocortical dynamics during whisker-based sensory discrimination in head-restrained mice.Neuroscience. 2018 Jan 1;368:57-69. doi: 10.1016/j.neuroscience.2017.09.003. Epub 2017 Sep 14. Neuroscience. 2018. PMID: 28919043 Free PMC article. Review.
Cited by
-
Brain-wide presynaptic networks of functionally distinct cortical neurons.Nature. 2025 May;641(8061):162-172. doi: 10.1038/s41586-025-08631-w. Epub 2025 Feb 26. Nature. 2025. PMID: 40011781 Free PMC article.
-
Feature-specific inhibitory connectivity augments the accuracy of cortical representations.bioRxiv [Preprint]. 2025 Aug 2:2025.08.02.668307. doi: 10.1101/2025.08.02.668307. bioRxiv. 2025. PMID: 40766410 Free PMC article. Preprint.
-
Perceptual choice and motor signals in mouse somatosensory cortex.bioRxiv [Preprint]. 2024 Dec 12:2024.12.06.627272. doi: 10.1101/2024.12.06.627272. bioRxiv. 2024. PMID: 39713341 Free PMC article. Preprint.
-
Brain-wide coordination of decision formation and commitment.bioRxiv [Preprint]. 2025 Aug 7:2024.08.21.609044. doi: 10.1101/2024.08.21.609044. bioRxiv. 2025. PMID: 40766510 Free PMC article. Preprint.
-
A fluid-walled microfluidic platform for human neuron microcircuits and directed axotomy.Lab Chip. 2024 Jun 25;24(13):3252-3264. doi: 10.1039/d4lc00107a. Lab Chip. 2024. PMID: 38841815 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

