A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory
- PMID: 36042313
- PMCID: PMC10614133
- DOI: 10.1038/s41593-022-01148-9
A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory
Abstract
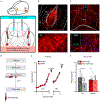
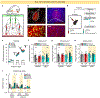
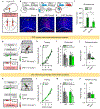
Behavioral flexibility-that is, the ability to deviate from established behavioral sequences-is critical for navigating dynamic environments and requires the durable encoding and retrieval of new memories to guide future choice. The orbitofrontal cortex (OFC) supports outcome-guided behaviors. However, the coordinated neural circuitry and cellular mechanisms by which OFC connections sustain flexible learning and memory remain elusive. Here we demonstrate in mice that basolateral amygdala (BLA)→OFC projections bidirectionally control memory formation when familiar behaviors are unexpectedly not rewarded, whereas OFC→dorsomedial striatum (DMS) projections facilitate memory retrieval. OFC neuronal ensembles store a memory trace for newly learned information, which appears to be facilitated by circuit-specific dendritic spine plasticity and neurotrophin signaling within defined BLA-OFC-DMS connections and obstructed by cocaine. Thus, we describe the directional transmission of information within an integrated amygdalo-fronto-striatal circuit across time, whereby novel memories are encoded by BLA→OFC inputs, represented within OFC ensembles and retrieved via OFC→DMS outputs during future choice.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures










Similar articles
-
Basolateral Amygdala to Orbitofrontal Cortex Projections Enable Cue-Triggered Reward Expectations.J Neurosci. 2017 Aug 30;37(35):8374-8384. doi: 10.1523/JNEUROSCI.0486-17.2017. Epub 2017 Jul 25. J Neurosci. 2017. PMID: 28743727 Free PMC article.
-
Effects of chronic ethanol exposure on dorsal medial striatal neurons receiving convergent inputs from the orbitofrontal cortex and basolateral amygdala.Neuropharmacology. 2025 Apr 1;267:110303. doi: 10.1016/j.neuropharm.2025.110303. Epub 2025 Jan 13. Neuropharmacology. 2025. PMID: 39814131
-
Amygdala-cortical collaboration in reward learning and decision making.Elife. 2022 Sep 5;11:e80926. doi: 10.7554/eLife.80926. Elife. 2022. PMID: 36062909 Free PMC article. Review.
-
The Orbitofrontal Cortex to Striatal Cholinergic Interneuron Circuit Controls Cognitive Flexibility Shaping Alcohol-Seeking Behavior.Biol Psychiatry. 2025 Mar 15;97(6):614-626. doi: 10.1016/j.biopsych.2024.10.005. Epub 2024 Oct 11. Biol Psychiatry. 2025. PMID: 39396737
-
Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making.J Neurosci. 2017 Nov 1;37(44):10529-10540. doi: 10.1523/JNEUROSCI.1678-17.2017. J Neurosci. 2017. PMID: 29093055 Free PMC article. Review.
Cited by
-
Dorsomedial Striatum (DMS) CB1R Signaling Promotes Pavlovian Devaluation Sensitivity in Male Long Evans Rats and Reduces DMS Inhibitory Synaptic Transmission in Both Sexes.eNeuro. 2025 Jan 29;12(1):ENEURO.0341-24.2024. doi: 10.1523/ENEURO.0341-24.2024. Print 2025 Jan. eNeuro. 2025. PMID: 39746803 Free PMC article.
-
Orbitofrontal cortex control of striatum leads economic decision-making.Nat Neurosci. 2023 Sep;26(9):1566-1574. doi: 10.1038/s41593-023-01409-1. Epub 2023 Aug 17. Nat Neurosci. 2023. PMID: 37592039 Free PMC article.
-
Neuronal Ensembles in the Amygdala Allow Social Information to Motivate Later Decisions.J Neurosci. 2024 Apr 17;44(16):e1848232024. doi: 10.1523/JNEUROSCI.1848-23.2024. J Neurosci. 2024. PMID: 38499360 Free PMC article.
-
Functional specialization of medial and lateral orbitofrontal cortex in inferential decision-making.iScience. 2024 May 17;27(6):110007. doi: 10.1016/j.isci.2024.110007. eCollection 2024 Jun 21. iScience. 2024. PMID: 38868183 Free PMC article.
-
Subunit-selective PI3-kinase control of action strategies in the medial prefrontal cortex.Neurobiol Learn Mem. 2023 Sep;203:107789. doi: 10.1016/j.nlm.2023.107789. Epub 2023 Jun 15. Neurobiol Learn Mem. 2023. PMID: 37328026 Free PMC article.
References
-
- Everitt BJ & Robbins TW Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol 67, 23–50 (2016). - PubMed
-
- Parkes SL et al. Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cereb. Cortex 28, 2313–2325 (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

