Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity
- PMID: 36042314
- PMCID: PMC9448679
- DOI: 10.1038/s41593-022-01140-3
Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity
Erratum in
-
Author Correction: Pathological structural conversion of α-synuclein at the mitochondria induces neuronal toxicity.Nat Neurosci. 2022 Nov;25(11):1582. doi: 10.1038/s41593-022-01206-2. Nat Neurosci. 2022. PMID: 36261654 Free PMC article. No abstract available.
Abstract
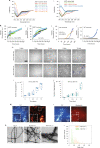
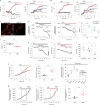
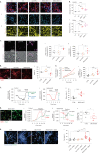
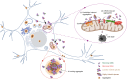
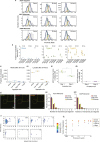
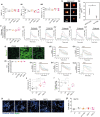
Aggregation of alpha-synuclein (α-Syn) drives Parkinson's disease (PD), although the initial stages of self-assembly and structural conversion have not been directly observed inside neurons. In this study, we tracked the intracellular conformational states of α-Syn using a single-molecule Förster resonance energy transfer (smFRET) biosensor, and we show here that α-Syn converts from a monomeric state into two distinct oligomeric states in neurons in a concentration-dependent and sequence-specific manner. Three-dimensional FRET-correlative light and electron microscopy (FRET-CLEM) revealed that intracellular seeding events occur preferentially on membrane surfaces, especially at mitochondrial membranes. The mitochondrial lipid cardiolipin triggers rapid oligomerization of A53T α-Syn, and cardiolipin is sequestered within aggregating lipid-protein complexes. Mitochondrial aggregates impair complex I activity and increase mitochondrial reactive oxygen species (ROS) generation, which accelerates the oligomerization of A53T α-Syn and causes permeabilization of mitochondrial membranes and cell death. These processes were also observed in induced pluripotent stem cell (iPSC)-derived neurons harboring A53T mutations from patients with PD. Our study highlights a mechanism of de novo α-Syn oligomerization at mitochondrial membranes and subsequent neuronal toxicity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
-
- Chartier-Harlin MC, et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. - PubMed
-
- Eliezer D, et al. Conformational properties of α-synuclein in its free and lipid-associated states. J. Mol. Biol. 2001;307:1061–1073. - PubMed
-
- Spillantini MG, et al. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Weinreb PH, et al. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

