Outcomes of Babies with Opioid Exposure (OBOE): protocol of a prospective longitudinal cohort study
- PMID: 36042329
- PMCID: PMC9971338
- DOI: 10.1038/s41390-022-02279-2
Outcomes of Babies with Opioid Exposure (OBOE): protocol of a prospective longitudinal cohort study
Erratum in
-
Correction To: Outcomes of Babies with Opioid Exposure (OBOE): protocol of a prospective longitudinal cohort study.Pediatr Res. 2023 Oct;94(4):1581. doi: 10.1038/s41390-023-02662-7. Pediatr Res. 2023. PMID: 37225780 Free PMC article. No abstract available.
Abstract
Background: While the health, social, and economic impacts of opioid addiction on adults and their communities are well known, the impact of maternal opioid use on the fetus exposed in utero is less well understood.
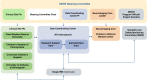
Methods: This paper presents the protocol of the ACT NOW Outcomes of Babies with Opioid Exposure (OBOE) Study, a multi-site prospective longitudinal cohort study of infants with antenatal opioid exposure and unexposed controls. Study objectives are to determine the impact of antenatal opioid exposure on brain development and neurodevelopmental outcomes over the first 2 years of life and explore whether family, home, and community factors modify developmental trajectories during this critical time period.
Results: Primary outcomes related to brain development include cortical volumes, deep cerebral gray matter volumes, resting-state functional connectivity measures, and structural connectivity measures using diffusion tensor imaging. Primary neurodevelopmental outcomes include visual abnormalities, cognitive, language, and motor skills measured using the Bayley Scales of Infant Development and social-emotional and behavioral problems and competence measured by the Brief Infant-Toddler Social and Emotional Assessment.
Conclusions: The OBOE study has been designed to overcome challenges of previous studies and will help further understanding of the effects of antenatal opioid exposure on early infant development.
Impact: This study will integrate MRI findings and comprehensive neurodevelopmental assessments to provide early insights into the functional topography of the brain in this high-risk population and assess MRI as a potential biomarker. Rather than conducting neuroimaging at a single time point, the study will include serial MRI assessments from birth to 2 years, allowing for the examination of trajectories throughout this period of rapid brain development. While previous studies often have had limited information on exposures, this study will use umbilical cord assays to accurately measure amounts of opioids and other substances from 20 weeks of gestation to birth.
© 2022. The Author(s), under exclusive licence to the International Pediatric Research Foundation, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Supplementing Consent for a Prospective Longitudinal Cohort Study of Infants With Antenatal Opioid Exposure: Development and Assessment of a Digital Tool.JMIR Form Res. 2025 Mar 4;9:e59954. doi: 10.2196/59954. JMIR Form Res. 2025. PMID: 40036491 Free PMC article.
-
Antenatal Opioid Exposure and Global and Regional Brain Volumes in Newborns.JAMA Pediatr. 2025 Jun 1;179(6):639-646. doi: 10.1001/jamapediatrics.2025.0277. JAMA Pediatr. 2025. PMID: 40193106
-
Lessons Learned in Virtual Launch of an Antenatal Opioid Exposure Study During the COVID-19 Pandemic.Nurs Res. 2025 May-Jun 01;74(3):218-224. doi: 10.1097/NNR.0000000000000807. Epub 2025 Jan 24. Nurs Res. 2025. PMID: 39853217 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
Cited by
-
Advances in the Care of Infants With Prenatal Opioid Exposure and Neonatal Opioid Withdrawal Syndrome.Pediatrics. 2024 Jan 1;153(2):e2023062871. doi: 10.1542/peds.2023-062871. Pediatrics. 2024. PMID: 38178779 Free PMC article. Review.
-
Trends in maternal opioid use: Statewide differences by sociodemographic characteristics in Florida from 2000 to 2019.J Addict Dis. 2024 Oct-Dec;42(4):524-534. doi: 10.1080/10550887.2024.2302285. Epub 2024 Feb 18. J Addict Dis. 2024. PMID: 38369773
-
Psychological distress among postpartum women who took opioids during pregnancy: the role of perceived stigma in healthcare settings.Arch Womens Ment Health. 2024 Apr;27(2):275-283. doi: 10.1007/s00737-023-01390-5. Epub 2023 Nov 13. Arch Womens Ment Health. 2024. PMID: 37955711 Free PMC article.
-
The immediate and long-term effects of prenatal opioid exposure.Front Pediatr. 2022 Nov 7;10:1039055. doi: 10.3389/fped.2022.1039055. eCollection 2022. Front Pediatr. 2022. PMID: 36419918 Free PMC article. Review.
-
Executive functioning, behavioural, emotional, and cognitive difficulties in school-aged children prenatally exposed to methadone.Front Pediatr. 2023 Apr 18;11:1118634. doi: 10.3389/fped.2023.1118634. eCollection 2023. Front Pediatr. 2023. PMID: 37144152 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

