The introduction of an N-glycosylation site into prochymosin greatly enhances its production and secretion by Pichia pastoris
- PMID: 36042512
- PMCID: PMC9429577
- DOI: 10.1186/s12934-022-01904-3
The introduction of an N-glycosylation site into prochymosin greatly enhances its production and secretion by Pichia pastoris
Abstract
Background: N-glycosylation is one of the most important post-translational modifications. Many studies have shown that N-glycosylation has a significant effect on the secretion level of heterologous glycoproteins in yeast cells. However, there have been few studies reporting a clear and unified explanation for the intracellular mechanism that N-glycosylation affect the secretion of heterologous glycoproteins so far. Pichia pastoris is an important microbial cell factory producing heterologous protein. It is of great significance to study the effect of N-glycosylation on the secretion level of heterologous protein. Camel chymosin is a glycoprotein with higher application potential in cheese manufacturing industry. We have expressed camel prochymosin in P. pastoris GS115, but the lower secretion level limits its industrial application. This study attempts to increase the secretion level of prochymosin through N-glycosylation, and explore the molecular mechanism of N-glycosylation affecting secretion.
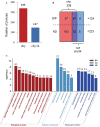
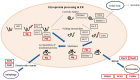
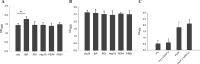
Results: Adding an N-glycosylation site at the 34th amino acid of the propeptide of prochymosin significantly increased its secretion in P. pastoris. N-glycosylation improved the thermostability of prochymosin without affecting the enzymatic activity. Immunoprecipitation coupled to mass spectrometry (IP-MS) analysis showed that compared with the wild prochymosin (chy), the number of proteins interacting with N-glycosylated mutant (chy34) decreased, and all differential interacting proteins (DIPs) were down-regulated in chy34-GS115 cell. The DIPs in endoplasmic reticulum were mainly concentrated in the misfolded protein pathway. Among the five DIPs in this pathway, overexpression of BiP significantly increased the secretion of chy. The knockout of the possible misfolded protein recognition elements, UDP-glycose:glycoprotein glucosyltransferase 1 and 2 (UGGT1/2) had no effect on the growth of yeast cells and the secretion of prochymosin.
Conclusions: In conclusion, N-glycosylation increased the secretion of prochymosin in P. pastoris trough the adjustment of intracellular interacted proteins. The results of our study may help to elucidate the molecular mechanism of N-glycosylation affecting secretion and provide a new research method to improve the secretion of heterologous glycoprotein in P. pastoris.
Keywords: Differential interacting proteins; N-glycosylation; Pichia pastoris; Prochymosin; Secretion.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Expression and characterization of camel chymosin in Pichia pastoris.Protein Expr Purif. 2015 Jul;111:75-81. doi: 10.1016/j.pep.2015.03.012. Epub 2015 Mar 31. Protein Expr Purif. 2015. PMID: 25837439
-
[Recombinant expression of bovine chymosin in Pichia pastoris].Sheng Wu Gong Cheng Xue Bao. 2009 Aug;25(8):1160-5. Sheng Wu Gong Cheng Xue Bao. 2009. PMID: 19938452 Chinese.
-
Optimizing Pichia pastoris protein secretion: Role of N-linked glycosylation on the α-mating factor secretion signal leader.J Biotechnol. 2024 Aug 10;391:1-10. doi: 10.1016/j.jbiotec.2024.04.008. Epub 2024 Apr 16. J Biotechnol. 2024. PMID: 38636846
-
Pathway engineering facilitates efficient protein expression in Pichia pastoris.Appl Microbiol Biotechnol. 2022 Sep;106(18):5893-5912. doi: 10.1007/s00253-022-12139-y. Epub 2022 Aug 30. Appl Microbiol Biotechnol. 2022. PMID: 36040488 Review.
-
Characterization of N-linked glycosylation on recombinant glycoproteins produced in Pichia pastoris using ESI-MS and MALDI-TOF.Methods Mol Biol. 2009;534:213-23. doi: 10.1007/978-1-59745-022-5_16. Methods Mol Biol. 2009. PMID: 19277549 Review.
Cited by
-
Komagataella phaffii as a Platform for Heterologous Expression of Enzymes Used for Industry.Microorganisms. 2024 Feb 7;12(2):346. doi: 10.3390/microorganisms12020346. Microorganisms. 2024. PMID: 38399750 Free PMC article. Review.
-
An impact of N-glycosylation on biochemical properties of a recombinant α-amylase from Bacillus licheniformis.Heliyon. 2024 Mar 13;10(6):e28064. doi: 10.1016/j.heliyon.2024.e28064. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38515717 Free PMC article.
References
-
- Adney WS, Jeoh T, Beckham GT, Chou YC, Baker JO, Michener W, Brunecky R, Himmel ME. Probing the role of N-linked glycans in the stability and activity of fungal cellobiohydrolases by mutational analysis. Cellulose. 2009;16:699–709. doi: 10.1007/s10570-009-9305-1. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

