Exosomal MicroRNA signature acts as an efficient biomarker for non-invasive diagnosis of gallbladder carcinoma
- PMID: 36043050
- PMCID: PMC9420508
- DOI: 10.1016/j.isci.2022.104816
Exosomal MicroRNA signature acts as an efficient biomarker for non-invasive diagnosis of gallbladder carcinoma
Abstract
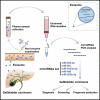
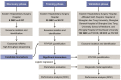
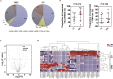
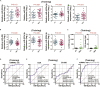
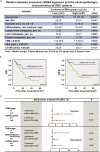
Through a three-step study that relies on biomarker discovery, training, and validation, we identified a set of five exosomal microRNAs (miRNAs) that can be used to evaluate the risk of gallbladder carcinoma (GBC), including miR-552-3p, miR-581, miR-4433a-3p, miR-496, and miR-203b-3p. When validated in 102 GBC patients and 112 chronic cholecystitis patients from multiple medical centers, the AUC of this combinatorial biomarker was 0.905, with a sensitivity of 81.37% and a specificity of 86.61%. The performance of this biomarker is superior to that of the standard biomarkers CA199 and CEA and is suited for GBC early diagnosis. The multi-clinicopathological features and prognosis of GBC patients were significantly associated with this biomarker. After building a miRNA-target gene regulation network, cell functions and signaling pathways regulated by these five miRNAs were examined. This biomarker signature can be used in the development of a noninvasive tool for GBC diagnosis, screening and prognosis prediction.
Keywords: Cancer; Cancer systems biology; Health sciences.
© 2022.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Brock G., Castellanos-Rizaldos E., Hu L., Coticchia C., Skog J. Liquid biopsy for cancer screening, patient stratification and monitoring. Transl. Cancer Res. 2015;4:280–290. doi: 10.3978/j.issn.2218-676X.2015.06.05. - DOI
-
- Dasari B.V.M., Ionescu M.I., Pawlik T.M., Hodson J., Sutcliffe R.P., Roberts K.J., Muiesan P., Isaac J., Marudanayagam R., Mirza D.F. Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: a systematic review and meta-analysis. J. Surg. Oncol. 2018;118:477–485. doi: 10.1002/jso.25186. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

