A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019
- PMID: 36043180
- PMCID: PMC9384635
- DOI: 10.1093/ofid/ofac406
A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019
Erratum in
-
Correction to: A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019.Open Forum Infect Dis. 2023 Apr 19;10(4):ofad196. doi: 10.1093/ofid/ofad196. eCollection 2023 Apr. Open Forum Infect Dis. 2023. PMID: 37089779 Free PMC article.
Abstract
Background: We evaluated clinical effectiveness of regdanvimab (CT-P59), a severe acute respiratory syndrome coronavirus 2 neutralizing monoclonal antibody, in reducing disease progression and clinical recovery time in patients with mild-to-moderate coronavirus disease 2019 (COVID-19), primarily Alpha variant.
Methods: This was phase 3 of a phase 2/3 parallel-group, double-blind, randomized clinical trial. Outpatients with mild-to-moderate COVID-19 were randomized to single-dose regdanvimab 40 mg/kg (n = 656) or placebo (n = 659), alongside standard of care. The primary endpoint was COVID-19 disease progression up to day 28 among "high-risk" patients. Key secondary endpoints were disease progression (all randomized patients) and time to recovery (high-risk and all randomized patients).
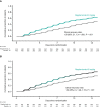
Results: Of 1315 randomized patients, 880 were high risk; the majority were infected with Alpha variant. The proportion with disease progression was lower (14/446, 3.1% [95% confidence interval {CI}, 1.9%-5.2%] vs 48/434, 11.1% [95% CI, 8.4%-14.4%]; P < .001) and time to recovery was shorter (median, 9.27 days [95% CI, 8.27-11.05 days] vs not reached [95% CI, 12.35-not calculable]; P < .001) with regdanvimab than placebo. Consistent improvements were seen in all randomized and non-high-risk patients who received regdanvimab. Viral load reductions were more rapid with regdanvimab. Infusion-related reactions occurred in 11 patients (4/652 [0.6%] regdanvimab, 7/650 [1.1%] placebo). Treatment-emergent serious adverse events were reported in 5 of (4/652 [0.6%] regdanvimab and 1/650 [0.2%] placebo).
Conclusions: Regdanvimab was an effective treatment for patients with mild-to-moderate COVID-19, significantly reducing disease progression and clinical recovery time without notable safety concerns prior to the emergence of the Omicron variant.
Clinical trials registration: NCT04602000; 2020-003369-20 (EudraCT).
Keywords: COVID-19 treatment; CT-P59; SARS-CoV-2; regdanvimab.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. Y. K. has been an investigator in COVID-19 clinical trials by Daewoong Pharmaceuticals, Enzychem Lifesciences, and GC Pharma, outside the scope of the submitted work, and by Celltrion, Inc, within the scope of the submitted work. O. S., An. S.-C., and Ad. S.-C. have been investigators in COVID-19 clinical trials by Algernon Pharmaceuticals, Atea Pharmaceuticals, Diffusion Pharmaceuticals, and Regeneron Pharmaceuticals, outside the scope of the submitted work, and by Celltrion, Inc, within the scope of the submitted work. L.-L. P., N. E. R.-M., M. D., V. B., E. G. M., N. G., O. C.-S., S.-A. F., and H. J. S. have been investigators in COVID-19 clinical trials by Celltrion, Inc, within the scope of the submitted work. S. J. L. and S. H. K. are employees of, and hold shares in, Celltrion, Inc. I. C., Y. J. B., J. H. S., D. R. C., S. J. K., M. R. K., S. G. L., and G. P. are employees of Celltrion, Inc. J. S. E. has been an investigator in COVID-19 clinical trials by Enzychem Lifesciences, Bukwang Pharm. Co., Ltd, and SK Chemicals outside the scope of the submitted work, and by Celltrion, Inc, within the scope of the submitted work.
Figures

References
-
- World Health Organization . Coronavirus disease (COVID-19) dashboard. https://covid19.who.int/. Accessed 13 June 2022.
-
- World Health Organization . Living guidance for clinical management of COVID-19. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed 3 May 2022. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

