Outcomes and Prognostic Factors of Metastatic Gastric Cancer: A Single-Center Experience
- PMID: 36043200
- PMCID: PMC9414169
- DOI: 10.7759/cureus.28426
Outcomes and Prognostic Factors of Metastatic Gastric Cancer: A Single-Center Experience
Abstract
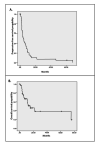
Background Gastric cancer (GC) carries a poor survival outcome despite the availability of many therapeutic agents active in treatment. In this study, we aimed to evaluate the survival outcomes of metastatic GC treatment from a single center in Saudi Arabia and identify possible prognostic factors. Methodology Data on patients diagnosed with metastatic GC between December 2009 and November 2013 were collected and analyzed. Results During this period, 41 patients were diagnosed with a median age at diagnosis of 52 years, and 56.1% of patients were males. Only four (9.2%) patients had human epidermal growth factor receptor 2 overexpression. Overall, 83% were treated with oxaliplatin-based chemotherapy. The median progression-free survival (PFS) and overall survival (OS) were 4.1 and 15.4 months, respectively. Female sex was an independent prognostic factor for better PFS and OS. Normal lymphocyte count was associated with improved PFS. Conclusions Our study highlights poor outcomes in patients with metastatic GC and the need for further research in this field.
Keywords: cancer survival; gastric cancer; gastroesophageal cancer; metastatic gastric cancer; progression-free survival.
Copyright © 2022, Aseafan et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Gastric cancer: epidemiology, biology, and prevention: a mini review. Lyons K, Le LC, Pham YT, et al. Eur J Cancer Prev. 2019;28:397–412. - PubMed
-
- Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Bang YJ, Van Cutsem E, Feyereislova A, et al. Lancet. 2010;376:687–697. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous