Alarm cues and alarmed conspecifics: neural activity during social learning from different cues in Trinidadian guppies
- PMID: 36043284
- PMCID: PMC9428528
- DOI: 10.1098/rspb.2022.0829
Alarm cues and alarmed conspecifics: neural activity during social learning from different cues in Trinidadian guppies
Abstract
Learning to respond appropriately to novel dangers is often essential to survival and success, but carries risks. Learning about novel threats from others (social learning) can reduce these risks. Many species, including the Trinidadian guppy (Poecilia reticulata), respond defensively to both conspecific chemical alarm cues and conspecific anti-predator behaviours, and in other fish such social information can lead to a learned aversion to novel threats. However, relatively little is known about the neural substrates underlying social learning and the degree to which different forms of learning share similar neural mechanisms. Here, we explored the neural substrates mediating social learning of novel threats from two different conspecific cues (i.e. social cue-based threat learning). We first demonstrated that guppies rapidly learn about threats paired with either alarm cues or with conspecific threat responses (demonstration). Then, focusing on acquisition rather than recall, we discovered that phospho-S6 expression, a marker of neural activity, was elevated in guppies during learning from alarm cues in the putative homologue of the mammalian lateral septum and the preoptic area. Surprisingly, these changes in neural activity were not observed in fish learning from conspecific demonstration. Together, these results implicate forebrain areas in social learning about threat but raise the possibility that circuits contribute to such learning in a stimulus-specific manner.
Keywords: anti-predator behaviour; conditioned threat learning; fear conditioning; pS6; social information; social learning.
Conflict of interest statement
The authors declare no competing interests.
Figures

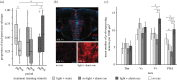
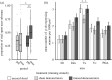
References
-
- Gill SA, Bierema AM-K. 2013. On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119, 449-461. (10.1111/eth.12097) - DOI
-
- McRae T. 2020. A review of squirrel alarm-calling behavior: what we know and what we do not know about how predator attributes affect alarm calls. Anim. Behav. Cogn. 7, 168-191. (10.26451/abc.07.02.11.2020) - DOI
-
- Chivers DP, Smith RJF. 1997. Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Écoscience 5, 338-352. (10.1080/11956860.1998.11682471) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

