Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer
- PMID: 36043530
- PMCID: PMC9561607
- DOI: 10.1158/1078-0432.CCR-22-0619
Clinical Validation of EndoPredict in Pre-Menopausal Women with ER-Positive, HER2-Negative Primary Breast Cancer
Abstract
Purpose: The EndoPredict prognostic assay is validated to predict distant recurrence and response to chemotherapy primarily in post-menopausal women with estrogen receptor-positive (ER+), HER2- breast cancer. This study evaluated the performance of EndoPredict in pre-menopausal women.
Experimental design: Tumor samples from 385 pre-menopausal women with ER+, HER2- primary breast cancer (pT1-3, pN0-1) who did not receive chemotherapy in addition to endocrine therapy were tested with EndoPredict to produce a 12-gene EP molecular score and an integrated EPclin score that includes pathologic tumor size and nodal status. Associations of molecular and EPclin scores with 10-year distant recurrence-free survival (DRFS) were evaluated by Cox proportional hazards models and Kaplan-Meier analysis.
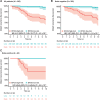
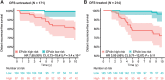
Results: After a median follow-up of 9.7 years, both the EP molecular score and the molecular-clinicopathologic EPclin score were associated with increased risk of distant recurrence [HR, 1.33; 95% confidence interval (CI), 1.18-1.50; P = 7.2 × 10-6; HR, 3.58; 95% CI, 2.26-5.66; P = 9.8 × 10-8, respectively]. Both scores remained significant after adjusting for clinical factors in multivariate analysis. Patients with low-risk EPclin scores (64.7%) had significantly improved DRFS compared with high-risk patients (HR, 4.61; 95% CI, 1.40-15.17; P = 4.2 × 10-3). At 10 years, patients with low-risk and high-risk EPclin scores had a DRFS of 97% (95% CI, 93%-99%) and 76% (95% CI, 67%-82%), respectively.
Conclusions: The EPclin score is strongly associated with DRFS in pre-menopausal women who received adjuvant endocrine therapy alone. On the basis of these data, pre-menopausal women with EPclin low-risk breast cancer may be treated with endocrine therapy only and safely forgo adjuvant chemotherapy.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18:452–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

