Sensory signals of unloading in insects are tuned to distinguish leg slipping from load variations in gait: experimental and modeling studies
- PMID: 36043841
- PMCID: PMC9529259
- DOI: 10.1152/jn.00285.2022
Sensory signals of unloading in insects are tuned to distinguish leg slipping from load variations in gait: experimental and modeling studies
Abstract
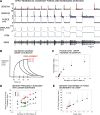
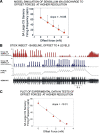
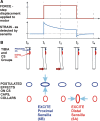
In control of walking, sensory signals of decreasing forces are used to regulate leg lifting in initiation of swing and to detect loss of substrate grip (leg slipping). We used extracellular recordings in two insect species to characterize and model responses to force decrements of tibial campaniform sensilla, receptors that detect forces as cuticular strains. Discharges to decreasing forces did not occur upon direct stimulation of the sites of mechanotransduction (cuticular caps) but were readily elicited by bending forces applied to the leg. Responses to bending force decreases were phasic but had rate sensitivities similar to discharges elicited by force increases in the opposite direction. Application of stimuli of equivalent amplitude at different offset levels showed that discharges were strongly dependent upon the tonic level of loading: firing was maximal to complete unloading of the leg but substantially decreased or eliminated by sustained loads. The contribution of cuticle properties to sensory responses was also evaluated: discharges to force increases showed decreased adaptation when mechanical stress relaxation was minimized; firing to force decreases could be related to viscoelastic "creep" in the cuticle. Discharges to force decrements apparently occur due to cuticle viscoelasticity that generates transient strains similar to bending in the opposite direction. Tuning of sensory responses through cuticular and membrane properties effectively distinguishes loss of substrate grip/complete unloading from force variations due to gait in walking. We have successfully reproduced these properties in a mathematical model of the receptors. Sensors with similar tuning could fulfil these functions in legs of walking machines.NEW & NOTEWORTHY Decreases in loading of legs are important in the regulation of posture and walking in both vertebrates and invertebrates. Recordings of activities of tibial campaniform sensilla, which encode forces in insects, showed that their responses are specifically tuned to detect force decreases at the end of the stance phase of walking or when a leg slips. These results have been reproduced in a mathematical model of the receptors and also have potential applications in robotics.
Keywords: posture; sensory encoding; unloading; walking.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Ansgar Büschges is an editor of
Figures




Similar articles
-
Mechanosensory encoding of forces in walking uphill and downhill: force feedback can stabilize leg movements in stick insects.J Neurophysiol. 2024 Feb 1;131(2):198-215. doi: 10.1152/jn.00414.2023. Epub 2024 Jan 3. J Neurophysiol. 2024. PMID: 38166479 Free PMC article.
-
Force sensing in small animals: recording response properties and modeling of tibial campaniform sensilla in blow flies.J Neurophysiol. 2025 Jun 1;133(6):1749-1760. doi: 10.1152/jn.00044.2025. Epub 2025 May 7. J Neurophysiol. 2025. PMID: 40331748
-
Evaluation of force feedback in walking using joint torques as "naturalistic" stimuli.J Neurophysiol. 2021 Jul 1;126(1):227-248. doi: 10.1152/jn.00120.2021. Epub 2021 Jun 9. J Neurophysiol. 2021. PMID: 34107221 Free PMC article.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
The scolopidial accessory organs and Nebenorgans in orthopteroid insects: Comparative neuroanatomy, mechanosensory function, and evolutionary origin.Arthropod Struct Dev. 2017 Nov;46(6):765-776. doi: 10.1016/j.asd.2017.08.004. Epub 2017 Sep 19. Arthropod Struct Dev. 2017. PMID: 28864301 Review.
Cited by
-
Mechanosensory encoding of forces in walking uphill and downhill: force feedback can stabilize leg movements in stick insects.J Neurophysiol. 2024 Feb 1;131(2):198-215. doi: 10.1152/jn.00414.2023. Epub 2024 Jan 3. J Neurophysiol. 2024. PMID: 38166479 Free PMC article.
-
Adaptive load feedback robustly signals force dynamics in robotic model of Carausius morosus stepping.Front Neurorobot. 2023 Jan 26;17:1125171. doi: 10.3389/fnbot.2023.1125171. eCollection 2023. Front Neurorobot. 2023. PMID: 36776993 Free PMC article.
-
Innate visual attraction before, during and after escape from adverse substrates in carpenter ants.J Exp Biol. 2025 Jul 1;228(13):jeb250278. doi: 10.1242/jeb.250278. Epub 2025 Jul 8. J Exp Biol. 2025. PMID: 40554759 Free PMC article.
-
A parametric finite element model of leg campaniform sensilla in Drosophila to study campaniform sensilla location and arrangement.J R Soc Interface. 2025 May;22(226):20240559. doi: 10.1098/rsif.2024.0559. Epub 2025 May 7. J R Soc Interface. 2025. PMID: 40329924
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

