Raptinal Induces Gasdermin E-Dependent Pyroptosis in Naïve and Therapy-Resistant Melanoma
- PMID: 36044013
- PMCID: PMC9722513
- DOI: 10.1158/1541-7786.MCR-22-0040
Raptinal Induces Gasdermin E-Dependent Pyroptosis in Naïve and Therapy-Resistant Melanoma
Abstract
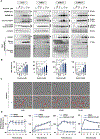
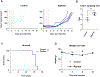
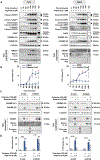
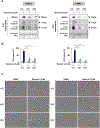
Lack of response and acquired resistance continue to be limitations of targeted and immune-based therapies. Pyroptosis is an inflammatory form of cell death characterized by the release of inflammatory damage-associated molecular patterns (DAMP) and cytokines via gasdermin (GSDM) protein pores in the plasma membrane. Induction of pyroptosis has implications for treatment strategies in both therapy-responsive, as well as resistance forms of melanoma. We show that the caspase-3 activator, raptinal, induces pyroptosis in both human and mouse melanoma cell line models and delays tumor growth in vivo. Release of DAMPs and inflammatory cytokines was dependent on caspase activity and GSDME expression. Furthermore, raptinal stimulated pyroptosis in melanoma models that have acquired resistance to BRAF and MEK inhibitor therapy. These findings add support to efforts to induce pyroptosis in both the treatment-naïve and resistant settings.
Implications: Raptinal can rapidly induce pyroptosis in naïve and BRAFi plus MEKi-resistant melanoma, which may be beneficial for patients who have developed acquired resistance to targeted therapies.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
Mutant BRAF and MEK Inhibitors Regulate the Tumor Immune Microenvironment via Pyroptosis.Cancer Discov. 2020 Feb;10(2):254-269. doi: 10.1158/2159-8290.CD-19-0672. Epub 2019 Dec 3. Cancer Discov. 2020. PMID: 31796433 Free PMC article.
-
TNFSF15 inhibits progression of diabetic retinopathy by blocking pyroptosis via interacting with GSDME.Cell Death Dis. 2024 Feb 8;15(2):118. doi: 10.1038/s41419-024-06499-8. Cell Death Dis. 2024. PMID: 38331883 Free PMC article.
-
Gasdermin E is required for induction of pyroptosis and severe disease during enterovirus 71 infection.J Biol Chem. 2022 May;298(5):101850. doi: 10.1016/j.jbc.2022.101850. Epub 2022 Mar 23. J Biol Chem. 2022. PMID: 35339492 Free PMC article.
-
Gasdermin D in pyroptosis.Acta Pharm Sin B. 2021 Sep;11(9):2768-2782. doi: 10.1016/j.apsb.2021.02.006. Epub 2021 Apr 28. Acta Pharm Sin B. 2021. PMID: 34589396 Free PMC article. Review.
-
Mini-Review: GSDME-Mediated Pyroptosis in Diabetic Nephropathy.Front Pharmacol. 2021 Nov 16;12:780790. doi: 10.3389/fphar.2021.780790. eCollection 2021. Front Pharmacol. 2021. PMID: 34867412 Free PMC article. Review.
Cited by
-
The Therapeutic Potential of Pyroptosis in Melanoma.Int J Mol Sci. 2023 Jan 9;24(2):1285. doi: 10.3390/ijms24021285. Int J Mol Sci. 2023. PMID: 36674798 Free PMC article. Review.
-
Role of gasdermin family proteins in cancers (Review).Int J Oncol. 2023 Sep;63(3):100. doi: 10.3892/ijo.2023.5548. Epub 2023 Jul 21. Int J Oncol. 2023. PMID: 37477150 Free PMC article.
-
Exploring the role of pyroptosis in the pathogenicity of heart disease.Front Physiol. 2024 Apr 5;15:1357285. doi: 10.3389/fphys.2024.1357285. eCollection 2024. Front Physiol. 2024. PMID: 38645692 Free PMC article. Review.
-
Melanoma biology and treatment: a review of novel regulated cell death-based approaches.Cancer Cell Int. 2024 Feb 9;24(1):63. doi: 10.1186/s12935-024-03220-9. Cancer Cell Int. 2024. PMID: 38336727 Free PMC article. Review.
-
Raptinal: a powerful tool for rapid induction of apoptotic cell death.Cell Death Discov. 2024 Aug 21;10(1):371. doi: 10.1038/s41420-024-02120-1. Cell Death Discov. 2024. PMID: 39164225 Free PMC article. Review.
References
-
- Boatright KM, Salvesen GS. Mechanisms of caspase activation. Current Opinion in Cell Biology. 2003;15:725–31. - PubMed
-
- Cullen SP, Martin SJ. Caspase activation pathways: some recent progress. Cell Death Differ. Nature Publishing Group; 2009;16:935–8. - PubMed
-
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. Nature Publishing Group; 2008;27:6194–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

