Inadequate Activation of γδT- and B-cells in Patient with Wiskott-Aldrich Syndrome (WAS) Portrayed by TRG and IGH Repertoire Analyses
- PMID: 36044170
- PMCID: PMC9840590
- DOI: 10.1007/s10875-022-01349-8
Inadequate Activation of γδT- and B-cells in Patient with Wiskott-Aldrich Syndrome (WAS) Portrayed by TRG and IGH Repertoire Analyses
Abstract
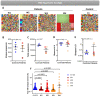
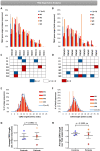
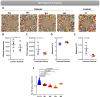
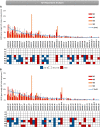
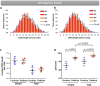
Patients with Wiskott-Aldrich syndrome (WAS) harbor mutations in the WAS gene and suffer from immunodeficiency, microthrombocytopenia, and eczema. T-cells play an important role in immune response in the skin and the γδT-cells have an important role in skin homeostasis. Since WAS patients often present with eczema, we wanted to examine whether the T-cell receptor gamma (TRG) repertoire of the γδT-cells is affected in these patients. In addition, the immunoglobulin heavy chain (IGH) repertoire from genomic DNA of WAS patients was not yet studied. Thus, we sought to determine the effects that specific WAS mutations from our patients have in shaping the TRG and IGH immune repertoires. We collected clinical and genetic data on four WAS patients, each harboring a different mutation in the WAS gene. Using next-generation sequencing (NGS), we analyzed their TRG and IGH repertoires using genomic DNA isolated from their peripheral blood. We analyzed the TRG and IGH repertoire sequences to show repertoire restriction, clonal expansions, preferential utilization of specific V genes, and unique characteristics of the antigen binding region in WAS patients with eczema compared to healthy controls. Both the TRG and IGH repertoire showed diverse repertoire comparable to healthy controls on one the hand, and on the other hand, the IGH repertoire showed increased diversity, more evenly distributed repertoire and immaturity of the antigen binding region. Thus, we demonstrate by analyzing the repertoire based on genomic DNA, the various effect that WAS mutations have in shaping the TRG and IGH adaptive immune repertoires.
Keywords: IGH repertoire; TRG repertoire; WAS; primary immunodeficiency.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

