Assessment of Clinical and Virological Characteristics of SARS-CoV-2 Infection Among Children Aged 0 to 4 Years and Their Household Members
- PMID: 36044218
- PMCID: PMC9434363
- DOI: 10.1001/jamanetworkopen.2022.27348
Assessment of Clinical and Virological Characteristics of SARS-CoV-2 Infection Among Children Aged 0 to 4 Years and Their Household Members
Erratum in
-
Error in Discussion.JAMA Netw Open. 2022 Sep 1;5(9):e2235523. doi: 10.1001/jamanetworkopen.2022.35523. JAMA Netw Open. 2022. PMID: 36107434 Free PMC article. No abstract available.
Abstract
Importance: Few studies have prospectively assessed SARS-CoV-2 community infection in children aged 0 to 4 years. Information about SARS-CoV-2 incidence and clinical and virological features in young children could help guide prevention and mitigation strategies.
Objective: To assess SARS-CoV-2 incidence, clinical and virological features, and symptoms in a prospective household cohort and to compare viral load by age group, symptoms, and SARS-CoV-2 lineage in young children, older children, and adults.
Design, setting, and participants: This prospective cohort study enrolled 690 participants from 175 Maryland households with 1 or more children aged 0 to 4 years between November 24, 2020, and October 15, 2021. For 8 months after enrollment, participants completed weekly symptom questionnaires and submitted self-collected nasal swabs for SARS-CoV-2 qualitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing, quantitative RT-PCR testing, and viral lineage determination. For the analyses, SARS-CoV-2 Alpha and Delta lineages were considered variants of interest or concern. Sera collected at enrollment and at approximately 4 months and 8 months after enrollment were assayed for SARS-CoV-2 spike and nucleocapsid protein antibodies.
Main outcomes and measures: Incidence, clinical and virological characteristics, and symptoms of SARS-CoV-2 infection by age group and correlations between (1) highest detected viral load and symptom frequency and (2) highest detected viral load and SARS-CoV-2 lineage.
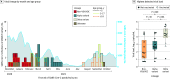
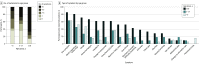
Results: Among 690 participants (355 [51.4%] female and 335 [48.6%] male), 256 individuals (37.1%) were children aged 0 to 4 years, 100 (14.5%) were children aged 5 to 17 years, and 334 (48.4%) were adults aged 18 to 74 years. A total of 15 participants (2.2%) were Asian, 24 (3.5%) were Black, 603 (87.4%) were White, 43 (6.2%) were multiracial, and 5 (0.7%) were of other races; 33 participants (4.8%) were Hispanic, and 657 (95.2%) were non-Hispanic. Overall, 54 participants (7.8%) had SARS-CoV-2 infection during the surveillance period, including 22 of 256 children (8.6%) aged 0 to 4 years, 11 of 100 children (11.0%) aged 5 to 17 years, and 21 of 334 adults (6.3%). Incidence rates per 1000 person-weeks were 2.25 (95% CI, 1.28-3.65) infections among children aged 0 to 4 years, 3.48 (95% CI, 1.59-6.61) infections among children aged 5 to 17 years, and 1.08 (95% CI, 0.52-1.98) infections among adults. Children aged 0 to 17 years with SARS-CoV-2 infection were more frequently asymptomatic (11 of 30 individuals [36.7%]) compared with adults (3 of 21 individuals [14.3%]), with children aged 0 to 4 years most frequently asymptomatic (7 of 19 individuals [36.8%]). The highest detected viral load did not differ between asymptomatic vs symptomatic individuals overall (median [IQR], 2.8 [1.5-3.3] log10 copies/mL vs 2.8 [1.8-4.4] log10 copies/mL) or by age group (median [IQR] for ages 0-4 years, 2.7 [2.4-4.4] log10 copies/mL; ages 5-17 years: 2.4 [1.1-4.0] log10 copies/mL; ages 18-74 years: 2.9 [1.9-4.6] log10 copies/mL). The number of symptoms was significantly correlated with viral load among adults (R = 0.69; P < .001) but not children (ages 0-4 years: R = 0.02; P = .91; ages 5-17 years: R = 0.18; P = .58). The highest detected viral load was greater among those with Delta variant infections (median [IQR], 4.4 [3.9-5.1] log10 copies/mL) than those with infections from variants not of interest or concern (median [IQR], 1.9 [1.1-3.6] log10 copies/mL; P = .009) or those with Alpha variant infections (median [IQR], 2.6 [2.3-3.4] log10 copies/mL; P = .006).
Conclusions and relevance: In this study, SARS-CoV-2 infections were frequently asymptomatic among children aged 0 to 4 years; the presence and number of symptoms did not correlate with viral load. These findings suggest that symptom screening may be insufficient to prevent outbreaks involving young children.
Conflict of interest statement
Figures

Comment in
-
How Do We Stop the Spread of SARS-CoV-2 in Young Children?JAMA Netw Open. 2022 Aug 1;5(8):e2227357. doi: 10.1001/jamanetworkopen.2022.27357. JAMA Netw Open. 2022. PMID: 36044224 No abstract available.
References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

