Nanosensors Based on a Single ZnO:Eu Nanowire for Hydrogen Gas Sensing
- PMID: 36044354
- PMCID: PMC9753046
- DOI: 10.1021/acsami.2c10975
Nanosensors Based on a Single ZnO:Eu Nanowire for Hydrogen Gas Sensing
Abstract
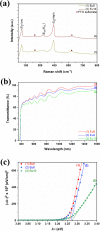
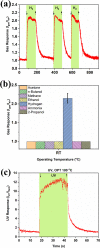
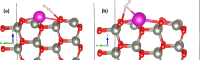
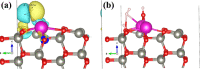
Fast detection of hydrogen gas leakage or its release in different environments, especially in large electric vehicle batteries, is a major challenge for sensing applications. In this study, the morphological, structural, chemical, optical, and electronic characterizations of ZnO:Eu nanowire arrays are reported and discussed in detail. In particular, the influence of different Eu concentrations during electrochemical deposition was investigated together with the sensing properties and mechanism. Surprisingly, by using only 10 μM Eu ions during deposition, the value of the gas response increased by a factor of nearly 130 compared to an undoped ZnO nanowire and we found an H2 gas response of ∼7860 for a single ZnO:Eu nanowire device. Further, the synthesized nanowire sensors were tested with ultraviolet (UV) light and a range of test gases, showing a UV responsiveness of ∼12.8 and a good selectivity to 100 ppm H2 gas. A dual-mode nanosensor is shown to detect UV/H2 gas simultaneously for selective detection of H2 during UV irradiation and its effect on the sensing mechanism. The nanowire sensing approach here demonstrates the feasibility of using such small devices to detect hydrogen leaks in harsh, small-scale environments, for example, stacked battery packs in mobile applications. In addition, the results obtained are supported through density functional theory-based simulations, which highlight the importance of rare earth nanoparticles on the oxide surface for improved sensitivity and selectivity of gas sensors, even at room temperature, thereby allowing, for instance, lower power consumption and denser deployment.
Keywords: Eu2O3; ZnO; electrochemical deposition; hydrogen; sensor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Saeedmanesh A.; Mac Kinnon M. A.; Brouwer J. Hydrogen Is Essential for Sustainability. Curr. Opin. Electrochem. 2018, 12, 166–181. 10.1016/j.coelec.2018.11.009. - DOI
-
- Arshad F.; Haq T.; Hussain I.; Sher F. Recent Advances in Electrocatalysts toward Alcohol-Assisted, Energy-Saving Hydrogen Production. ACS Appl. Energy Mater. 2021, 4, 8685–8701. 10.1021/acsaem.1c01932. - DOI
-
- Ohsaki T.; Kishi T.; Kuboki T.; Takami N.; Shimura N.; Sato Y.; Sekino M.; Satoh A. Overcharge Reaction of Lithium-Ion Batteries. J. Power Sources 2005, 146, 97–100. 10.1016/j.jpowsour.2005.03.105. - DOI
-
- Essl C.; Golubkov A. W.; Fuchs A. Comparing Different Thermal Runaway Triggers for Two Automotive Lithium-Ion Battery Cell Types. J. Electrochem. Soc. 2020, 167, 130542.10.1149/1945-7111/abbe5a. - DOI
-
- Guide for the Ventilation and Thermal Management of Batteries for Stationary Applications; IEEE, 2018.

