An optimized method of extracting and quantifying active Neutrophil serine proteases from human whole blood cells
- PMID: 36044421
- PMCID: PMC9432755
- DOI: 10.1371/journal.pone.0272575
An optimized method of extracting and quantifying active Neutrophil serine proteases from human whole blood cells
Abstract
Purpose: Neutrophil serine proteases (NSPs) are implicated in numerous inflammatory diseases. Thus, a robust methodology to monitor and quantify NSPs is important to study disease progression and evaluate the effect of pharmacological interventions. A comparison of the various methods used to extract NSPs from neutrophil granulocytes has not been published, providing the impetus to conduct this method optimization and comparison study.
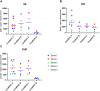
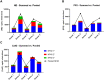
Methods: Two NSP recovery methodologies were evaluated on samples from five human donors: zymosan stimulation and cell pellet extraction. For the zymosan stimulation method, 1 mL donor blood was added to zymosan and samples were incubated at 37°C for 30 min while shaking. Samples were then centrifuged, and the plasma was collected for quantitation of NSP activity. For the cell pellet extraction procedure, 2 mL whole blood samples were centrifuged into white blood cell pellets following red blood cell lysis. To each pellet, three sequential lysis steps were performed using either 0.05% Nonidet P-40 Substitute (NP40) or 0.02% Triton X-100 lysis buffers under agitation followed by centrifugation. NSP activities were quantified using an exogenous peptide substrate specific to each of the three NSPs being analyzed: neutrophil elastase, cathepsin G, and proteinase 3.
Results and discussion: The zymosan stimulation method resulted in lower recovery of active NSPs and was unable to stimulate significant release of active cathepsin G. In contrast, the NP40 pellet extraction method showed consistent inter-donor NSP release with greater recoveries of active NSPs than the Triton method or the zymosan stimulation method. Overall, the pellet extraction procedure provided 13.3-fold greater recovery of active neutrophil elastase, 283-fold greater recovery of active cathepsin G, and 2.9-fold greater recovery of active proteinase 3 than the zymosan method.
Conclusion: The NP40 cell pellet extraction method resulted in greater extraction of active NSPs compared to the other methods investigated here, which may allow for a more accurate and complete biomarker profile when evaluating human clinical samples.
Conflict of interest statement
Authors JB, JZ, DL, SR, KJC, and DC were salaried employees of Insmed Incorporated during the course of the study. The authors would like to declare that a patent application associated with this research has been filed by Insmed Incorporated and has been published as WO 2022/020245 with JB, JZ and DC as inventors. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

