Engineering Catalytically Self-Sufficient P450s
- PMID: 36044428
- PMCID: PMC11107884
- DOI: 10.1021/acs.biochem.2c00336
Engineering Catalytically Self-Sufficient P450s
Abstract
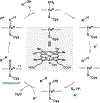
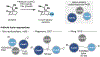
The P450 superfamily comprises some of the most powerful and versatile enzymes for the site-selective oxidation of small molecules. One of the main drawbacks for the applications of the P450s in biotechnology is that the majority of these enzymes is multicomponent in nature and requires the presence of suitable redox partners to support their functions. Nevertheless, the discovery of several self-sufficient P450s, namely those from Classes VII and VIII, has served as an inspiration for fusion approaches to generate chimeric P450 systems that are self-sufficient. In this Perspective, we highlight the domain organizations of the Class VII and Class VIII P450 systems, summarize recent case studies in the engineering of catalytically self-sufficient P450s based on these systems, and outline outstanding challenges in the field, along with several emerging technologies as potential solutions.
Figures
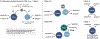

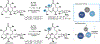


References
-
- Poulos TL; Johnson EF Structures of Cytochrome P450 Enzymes, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. Ortiz de Montellano PR, (Springer, Switzerland, 2015) p. 3–32.
-
- Klingenberg M Pigments of rat liver microsomes. Arch. Biochem. Biophys 1958, 75, 376–386. - PubMed
-
- Guengerich FP Human Cytochrome P450 Enzymes, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. Ortiz de Montellano PR, (Springer, Switzerland, 2015) p. 523–786.
- Auchus RJ; Miller WL P450 Enzymes in Steroid Processing, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. Ortiz de Montellano PR, (Springer, Switzerland, 2015) p. 851–881.
-
- Podust LM; Sherman DH Diversity of P450 enzymes in the biosynthesis of natural products. Nat. Prod. Rep 2012, 29, 1251–1266. - PMC - PubMed
- Rudolf JD; Chang C-Y; Ma M; Shen B Cytochromes P450 for natural product biosynthesis in Streptomyces: sequence, structure, and function. Nat. Prod. Rep 2017, 34, 1141–1172. - PMC - PubMed
- Zhang X; Guo J; Cheng F; Li S Cytochrome P450 enzymes in fungal natural product biosynthesis. Nat. Prod. Rep 2021, 38, 1072–1099. - PubMed
-
- Fasan R Tuning P450 enzymes as oxidation catalysts. ACS Catal 2012, 2, 647–666.
- Urlacher VB; Girhard M Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol 2019, 37, 882–897. - PubMed
- Girvan HM; Munro AW Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology. Curr. Opin. Chem. Biol 2016, 31, 136–145. - PubMed
- Jung ST; Lauchli R; Arnold FH Cytochrome P450: taming a wild type enzyme. Curr. Opin. Biotechnol 2011, 22, 809–817. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

