Disruption of mitochondria-sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure
- PMID: 36044551
- PMCID: PMC9456763
- DOI: 10.1073/pnas.2206708119
Disruption of mitochondria-sarcoplasmic reticulum microdomain connectomics contributes to sinus node dysfunction in heart failure
Abstract
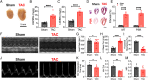
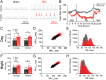
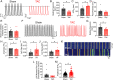
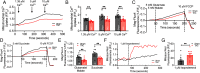
The sinoatrial node (SAN), the leading pacemaker region, generates electrical impulses that propagate throughout the heart. SAN dysfunction with bradyarrhythmia is well documented in heart failure (HF). However, the underlying mechanisms are not completely understood. Mitochondria are critical to cellular processes that determine the life or death of the cell. The release of Ca2+ from the ryanodine receptors 2 (RyR2) on the sarcoplasmic reticulum (SR) at mitochondria-SR microdomains serves as the critical communication to match energy production to meet metabolic demands. Therefore, we tested the hypothesis that alterations in the mitochondria-SR connectomics contribute to SAN dysfunction in HF. We took advantage of a mouse model of chronic pressure overload-induced HF by transverse aortic constriction (TAC) and a SAN-specific CRISPR-Cas9-mediated knockdown of mitofusin-2 (Mfn2), the mitochondria-SR tethering GTPase protein. TAC mice exhibited impaired cardiac function with HF, cardiac fibrosis, and profound SAN dysfunction. Ultrastructural imaging using electron microscope (EM) tomography revealed abnormal mitochondrial structure with increased mitochondria-SR distance. The expression of Mfn2 was significantly down-regulated and showed reduced colocalization with RyR2 in HF SAN cells. Indeed, SAN-specific Mfn2 knockdown led to alterations in the mitochondria-SR microdomains and SAN dysfunction. Finally, disruptions in the mitochondria-SR microdomains resulted in abnormal mitochondrial Ca2+ handling, alterations in localized protein kinase A (PKA) activity, and impaired mitochondrial function in HF SAN cells. The current study provides insights into the role of mitochondria-SR microdomains in SAN automaticity and possible therapeutic targets for SAN dysfunction in HF patients.
Keywords: bradycardia; heart failure; mitochondria; sinoatrial node; sinoatrial node dysfunction.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Metra M., Teerlink J. R., Heart failure. Lancet 390, 1981–1995 (2017). - PubMed
-
- Benjamin E. J., et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 137, e67–e492 (2018). - PubMed
-
- Sanders P., Kistler P. M., Morton J. B., Spence S. J., Kalman J. M., Remodeling of sinus node function in patients with congestive heart failure: Reduction in sinus node reserve. Circulation 110, 897–903 (2004). - PubMed
-
- Mangoni M. E., Nargeot J., Genesis and regulation of the heart automaticity. Physiol. Rev. 88, 919–982 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC004215/DC/NIDCD NIH HHS/United States
- R01 HL161872/HL/NHLBI NIH HHS/United States
- P01 AG051443/AG/NIA NIH HHS/United States
- R01 AG060504/AG/NIA NIH HHS/United States
- R01 DC015135/DC/NIDCD NIH HHS/United States
- R01 HL149127/HL/NHLBI NIH HHS/United States
- U01 HL160274/HL/NHLBI NIH HHS/United States
- F32 HL149288/HL/NHLBI NIH HHS/United States
- R01 HL137228/HL/NHLBI NIH HHS/United States
- R01 DC016099/DC/NIDCD NIH HHS/United States
- R01 HL085844/HL/NHLBI NIH HHS/United States
- R01 HL085727/HL/NHLBI NIH HHS/United States
- R01 HL155907/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

