Ventromedial hypothalamic OGT drives adipose tissue lipolysis and curbs obesity
- PMID: 36044565
- PMCID: PMC9432828
- DOI: 10.1126/sciadv.abn8092
Ventromedial hypothalamic OGT drives adipose tissue lipolysis and curbs obesity
Abstract
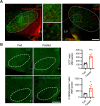
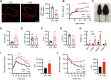
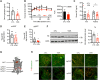
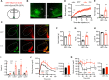
The ventromedial hypothalamus (VMH) is known to regulate body weight and counterregulatory response. However, how VMH neurons regulate lipid metabolism and energy balance remains unknown. O-linked β-d-N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation), catalyzed by O-GlcNAc transferase (OGT), is considered a cellular sensor of nutrients and hormones. Here, we report that genetic ablation of OGT in VMH neurons inhibits neuronal excitability. Mice with VMH neuron-specific OGT deletion show rapid weight gain, increased adiposity, and reduced energy expenditure, without significant changes in food intake or physical activity. The obesity phenotype is associated with adipocyte hypertrophy and reduced lipolysis of white adipose tissues. In addition, OGT deletion in VMH neurons down-regulates the sympathetic activity and impairs the sympathetic innervation of white adipose tissues. These findings identify OGT in the VMH as a homeostatic set point that controls body weight and underscore the importance of the VMH in regulating lipid metabolism through white adipose tissue-specific innervation.
Figures



References
-
- Morton G. J., Cummings D. E., Baskin D. G., Barsh G. S., Schwartz M. W., Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006). - PubMed
-
- Myers M. G. Jr., Olson D. P., Central nervous system control of metabolism. Nature 491, 357–363 (2012). - PubMed
-
- Schwartz M. W., Woods S. C., Porte D. Jr., Seeley R. J., Baskin D. G., Central nervous system control of food intake. Nature 404, 661–671 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

