Eribulin Treatment for Patients with Metastatic Breast Cancer: The UK Experience - A Multicenter Retrospective Study
- PMID: 36044833
- PMCID: PMC9808648
- DOI: 10.1159/000526140
Eribulin Treatment for Patients with Metastatic Breast Cancer: The UK Experience - A Multicenter Retrospective Study
Abstract
Introduction: This study examined real-world data from patients who received eribulin for metastatic breast cancer (MBC) collected from 14 hospitals across the UK.
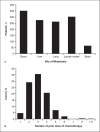
Methods: Anonymized data were collected retrospectively from patients with MBC who had received eribulin. The data included the hormone-receptor status, histological diagnosis, age, prior chemotherapy, response to eribulin, progression-free survival (PFS), and overall survival (OS).
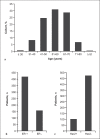
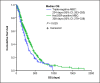
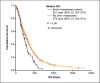
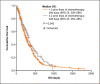
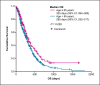
Results: Among 577 patients analyzed, the median age was 56 years, and most patients (73%) were estrogen-receptor positive. The median OS was 288 days (95% confidence interval [CI]: 261-315), and the PFS was 117 days (95% CI: 105-129). The median OS was higher among older patients (≥65 vs. <65 years: 325 days [95% CI: 264-385] vs. 285 days [95% CI: 252-317]; p = 0.028). The median OS was also higher in patients who received eribulin after fewer prior lines of chemotherapy (≤2 vs. >2 prior: 328 days [95% CI: 264-385] vs. 264 days [95% CI: 229-298]; p = 0.042).
Discussion/conclusion: These retrospective data suggest that eribulin can be successfully used in older patients with MBC. Eribulin treatment was more effective in earlier-line settings, which, while predictable, supports consideration of eribulin as a second-line treatment option.
Keywords: Eribulin; Metastatic breast cancer; Observation study; Older adults; Real-world data; Second-line treatment.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Dr. Mariam Jafri reports receiving personal fees from Eisai during the conduct of the study; personal fees from Roche, Pfizer, and Lilly, outside the submitted work. Dr. Samreen Ahmed reports receiving consultancy and advisory fees from Eisai. Dr. Annabel Borley reports personal fees from a commercial sponsor, outside the submitted work (i.e., an honorarium for participating in the Steering Committee for First Thoughts Educational Meeting 2018). Dr. Hartmut Kristeleit reports personal fees from Eisai during the conduct of the study; personal fees from Novartis and Roche; personal fees and other from Pfizer; and other from Daiichi-Sankyo, outside the submitted work. Dr. Vivek Misra reports personal fees from Eisai UK, outside the submitted work. Dr. Daniel Rea reports nonfinancial support from Eisai and Novartis; personal fees from Roche, Novartis, Pfizer, and Lily; grants from Roche, Biotheranostics, and RNA Diagnostics; and personal fees from Daiichi-Sankyo, outside the submitted work. Dr. Urmila Barthakur, Dr. Mark Baxter, Dr. Gwenllian Edwards, Dr. Ankit Jain, Dr. Apurna Jegannathen, Dr. Madeha Khan, Dr. David Maskell, Dr. Richard Walshaw, and Dr. Harriet S. Walter have nothing to disclose.
Figures
References
-
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10((Suppl 3)):20–9. - PubMed
-
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23((Suppl 7)):vii11–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

