Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers
- PMID: 36044963
- PMCID: PMC9420538
- DOI: 10.1016/j.ijid.2022.08.022
Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers
Abstract
Objectives: There are limited comparative immunologic durability data post COVID-19 vaccinations.
Methods: Approximately 8.4 months after primary COVID-19 vaccination, 647 healthcare workers completed surveys about COVID-19 vaccinations/infections and blood draws. The groups included participants vaccinated with mRNA-1273 (n = 387), BNT162b2 (n = 212), or Ad26.COV2.S (n = 10) vaccines; unvaccinated participants (n = 10); and participants who received a booster dose (n = 28). The primary outcome was immunoglobin anti-spike titer. Secondary/tertiary outcomes included neutralizing antibodies (enzyme-linked immunosorbent assay-based pseudoneutralization) and vaccine effectiveness (VE). Antibody levels were compared using analysis of variance and linear regression.
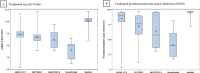
Results: Mean age was 49.7 and 75.3% of the participants were female. Baseline variables were balanced except for immunosuppression, previous COVID-19 infection, and post-primary vaccination time. Unadjusted median (interquartile range [IQR]) anti-spike titers (AU/ml) were 1539.5 (876.7-2626.7) for mRNA-1273, 751.2 (422.0-1381.5) for BNT162b2, 451.6 (103.0-2396.7) for Ad26.COV2.S, 113.4 (3.7-194.0) for unvaccinated participants, and 31898.8 (21347.1-45820.1) for participants administered with booster dose (mRNA-1273 vs BNT162b2, P <.001; mRNA-1273, BNT162b2, or boosted vs unvaccinated, P <.006; mRNA-1273, BNT162b2, Ad26.COV2.S, or unvaccinated vs boosted, P <.001). Unadjusted median (IQR) pseudoneutralization was as follows: 90.9% (80.1-95.0) for mRNA-1273, 77.2% (59.1-89.9) for BNT162b2, 57.9% (36.6-95.8) for Ad26.COV2.S, 40.1% (21.7-60.6) for unvaccinated, and 96.4% (96.1-96.6) for participants administered with booster dose (mRNA-1273 vs BNT162b2, P <.001; mRNA-1273, BNT162b2, or boosted vs unvaccinated, P <.028; mRNA-1273, BNT162b2, Ad26.COV2.S, or unvaccinated vs boosted, P <.001). VE was 87-89% for participants administered mRNA-1273 vaccine, BNT162b2 vaccine, and booster dose, and 33% for Ad26.COV2.S (none significantly different).
Conclusion: Antibody responses 8.4 months after primary vaccination were significantly higher with mRNA-1273 than those observed with BNT162b2.
Keywords: Anti-spike antibody; Antibody responses; COVID-19; SARS-CoV-2; Vaccine effectiveness.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest The authors have no competing interests to declare.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

