Biosynthesis of the Unusual Carbon Skeleton of Nocuolin A
- PMID: 36044983
- PMCID: PMC9486936
- DOI: 10.1021/acschembio.2c00464
Biosynthesis of the Unusual Carbon Skeleton of Nocuolin A
Abstract
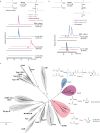
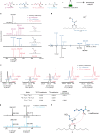
Nocuolin A is a cytotoxic cyanobacterial metabolite that is proposed to be produced by enzymes of the noc biosynthetic gene cluster. Nocuolin A features a 1,2,3-oxadiazine moiety, a structural feature unique among natural products and, so far, inaccessible through organic synthesis, suggesting that novel enzymatic chemistry might be involved in its biosynthesis. This heterocycle is substituted with two alkyl chains and a 3-hydroxypropanoyl moiety. We report here our efforts to elucidate the origin of the carbon skeleton of nocuolin A. Supplementation of cyanobacterial cultures with stable isotope-labeled fatty acids revealed that the central C13 chain is assembled from two medium-chain fatty acids, hexanoic and octanoic acids. Using biochemical assays, we show that a fatty acyl-AMP ligase, NocH, activates both fatty acids as acyl adenylates, which are loaded onto an acyl carrier protein domain and undergo a nondecarboxylative Claisen condensation catalyzed by the ketosynthase NocG. This enzyme is part of a phylogenetically well-defined clade within similar genomic contexts. NocG presents a unique combination of characteristics found in other ketosynthases, namely in terms of substrate specificity and reactivity. Further supplementation experiments indicate that the 3-hydroxypropanoyl moiety of 1 originates from methionine, through an as-yet-uncharacterized mechanism. This work provides ample biochemical evidence connecting the putative noc biosynthetic gene cluster to nocuolin A and identifies the origin of all its carbon atoms, setting the stage for elucidation of its unusual biosynthetic chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Burja A. M.; Banaigs B.; Abou-Mansour E.; Burgess J. G.; Wright P. C. Marine cyanobacteria - a prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. 10.1016/S0040-4020(01)00931-0. - DOI
-
- Tidgewell K.; Clark B. R.; Gerwick W. H., The Natural Products Chemistry of Cyanobacteria. Comprehensive Natural Products Ii: Chemistry and Biology, Vol 2: Natural Products Structural Diversity-Ii: Secondary Metabolites: Sources, Structures and Chemical Biology, 2010; pp 141–188.
Grants and funding
LinkOut - more resources
Full Text Sources

