Frustration and the Kinetic Repartitioning Mechanism of Substrate Inhibition in Enzyme Catalysis
- PMID: 36044985
- PMCID: PMC9483917
- DOI: 10.1021/acs.jpcb.2c03832
Frustration and the Kinetic Repartitioning Mechanism of Substrate Inhibition in Enzyme Catalysis
Abstract
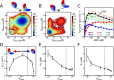
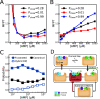
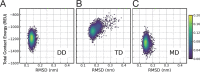
Substrate inhibition, whereby enzymatic activity decreases with excess substrate after reaching a maximum turnover rate, is among the most elusive phenomena in enzymatic catalysis. Here, based on a dynamic energy landscape model, we investigate the underlying mechanism by performing molecular simulations and frustration analysis for a model enzyme adenylate kinase (AdK), which catalyzes the phosphoryl transfer reaction ATP + AMP ⇋ ADP + ADP. Intriguingly, these reveal a kinetic repartitioning mechanism of substrate inhibition, whereby excess substrate AMP suppresses the population of an energetically frustrated, but kinetically activated, catalytic pathway going through a substrate (ATP)-product (ADP) cobound complex with steric incompatibility. Such a frustrated pathway plays a crucial role in facilitating the bottleneck product ADP release, and its suppression by excess substrate AMP leads to a slow down of product release and overall turnover. The simulation results directly demonstrate that substrate inhibition arises from the rate-limiting product-release step, instead of the steps for populating the catalytically competent complex as often suggested in previous works. Furthermore, there is a tight interplay between the enzyme conformational equilibrium and the extent of substrate inhibition. Mutations biasing to more closed conformations tend to enhance substrate inhibition. We also characterized the key features of single-molecule enzyme kinetics with substrate inhibition effect. We propose that the above molecular mechanism of substrate inhibition may be relevant to other multisubstrate enzymes in which product release is the bottleneck step.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Segel I. H.Enzyme Kinetics; Wiley: New York, 1993.
-
- Hill G. A.; Robinson C. W. Substrate inhibition kinetics: phenol degradation by Pseudomonas putida. Biotechnol. Bioeng. 1975, 17, 1599–1615. 10.1002/bit.260171105. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

