Dysregulated naive B cells and de novo autoreactivity in severe COVID-19
- PMID: 36044993
- PMCID: PMC9630115
- DOI: 10.1038/s41586-022-05273-0
Dysregulated naive B cells and de novo autoreactivity in severe COVID-19
Abstract
Severe SARS-CoV-2 infection1 has been associated with highly inflammatory immune activation since the earliest days of the COVID-19 pandemic2-5. More recently, these responses have been associated with the emergence of self-reactive antibodies with pathologic potential6-10, although their origins and resolution have remained unclear11. Previously, we and others have identified extrafollicular B cell activation, a pathway associated with the formation of new autoreactive antibodies in chronic autoimmunity12,13, as a dominant feature of severe and critical COVID-19 (refs. 14-18). Here, using single-cell B cell repertoire analysis of patients with mild and severe disease, we identify the expansion of a naive-derived, low-mutation IgG1 population of antibody-secreting cells (ASCs) reflecting features of low selective pressure. These features correlate with progressive, broad, clinically relevant autoreactivity, particularly directed against nuclear antigens and carbamylated proteins, emerging 10-15 days after the onset of symptoms. Detailed analysis of the low-selection compartment shows a high frequency of clonotypes specific for both SARS-CoV-2 and autoantigens, including pathogenic autoantibodies against the glomerular basement membrane. We further identify the contraction of this pathway on recovery, re-establishment of tolerance standards and concomitant loss of acute-derived ASCs irrespective of antigen specificity. However, serological autoreactivity persists in a subset of patients with postacute sequelae, raising important questions as to the contribution of emerging autoreactivity to continuing symptomology on recovery. In summary, this study demonstrates the origins, breadth and resolution of autoreactivity in severe COVID-19, with implications for early intervention and the treatment of patients with post-COVID sequelae.
© 2022. The Author(s).
Conflict of interest statement
F.E.L. is the founder of MicroB-plex, Inc., and has research grants with Genentech. M.E.R. is employed by Exagen, Inc. M.P. is employed by Nicoya.
Figures





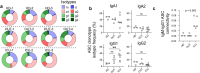

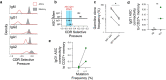
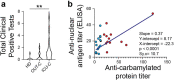



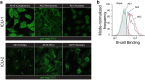
Update of
-
Relaxed peripheral tolerance drives broad de novo autoreactivity in severe COVID-19.medRxiv [Preprint]. 2021 Jul 27:2020.10.21.20216192. doi: 10.1101/2020.10.21.20216192. medRxiv. 2021. Update in: Nature. 2022 Nov;611(7934):139-147. doi: 10.1038/s41586-022-05273-0. PMID: 33106819 Free PMC article. Updated. Preprint.
References
-
- Chen X, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 EB021962/EB/NIBIB NIH HHS/United States
- 1R01AI12125/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R37 AI049660/AI/NIAID NIH HHS/United States
- R01 AI121252/AI/NIAID NIH HHS/United States
- P01 AI125180/AI/NIAID NIH HHS/United States
- T32-HL116271-07/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- U01 AI141993/AI/NIAID NIH HHS/United States
- U19-AI110483/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R37-AI049660/Division of Intramural Research, National Institute of Allergy and Infectious Diseases (Division of Intramural Research of the NIAID)
- 1U01AI141993/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P01-AI125180-01/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- INV-002351/GATES/Gates Foundation/United States
- U19 AI110483/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

