The whisking oscillator circuit
- PMID: 36045290
- PMCID: PMC10038238
- DOI: 10.1038/s41586-022-05144-8
The whisking oscillator circuit
Abstract
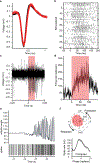
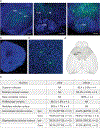
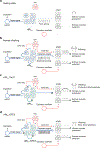
Central oscillators are primordial neural circuits that generate and control rhythmic movements1,2. Mechanistic understanding of these circuits requires genetic identification of the oscillator neurons and their synaptic connections to enable targeted electrophysiological recording and causal manipulation during behaviours. However, such targeting remains a challenge with mammalian systems. Here we delimit the oscillator circuit that drives rhythmic whisking-a motor action that is central to foraging and active sensing in rodents3,4. We found that the whisking oscillator consists of parvalbumin-expressing inhibitory neurons located in the vibrissa intermediate reticular nucleus (vIRtPV) in the brainstem. vIRtPV neurons receive descending excitatory inputs and form recurrent inhibitory connections among themselves. Silencing vIRtPV neurons eliminated rhythmic whisking and resulted in sustained vibrissae protraction. In vivo recording of opto-tagged vIRtPV neurons in awake mice showed that these cells spike tonically when animals are at rest, and transition to rhythmic bursting at the onset of whisking, suggesting that rhythm generation is probably the result of network dynamics, as opposed to intrinsic cellular properties. Notably, ablating inhibitory synaptic inputs to vIRtPV neurons quenched their rhythmic bursting, impaired the tonic-to-bursting transition and abolished regular whisking. Thus, the whisking oscillator is an all-inhibitory network and recurrent synaptic inhibition has a key role in its rhythmogenesis.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures










References
-
- Vincent SB The Functions of the Vibrissae in the Behavior of the White Rat. (University of Chicago, 1912).
-
- Welker W Analysis of sniffing of the albino rat 1. Behaviour 22, 223–244 (1964).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

