Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL
- PMID: 36045296
- PMCID: PMC9452296
- DOI: 10.1038/s41586-022-05140-y
Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL
Abstract
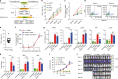
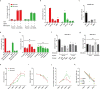
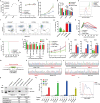
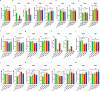
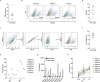
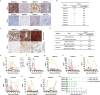
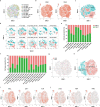
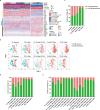
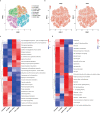
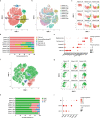
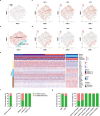
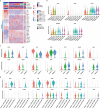
Recently, chimeric antigen receptor (CAR)-T cell therapy has shown great promise in treating haematological malignancies1-7. However, CAR-T cell therapy currently has several limitations8-12. Here we successfully developed a two-in-one approach to generate non-viral, gene-specific targeted CAR-T cells through CRISPR-Cas9. Using the optimized protocol, we demonstrated feasibility in a preclinical study by inserting an anti-CD19 CAR cassette into the AAVS1 safe-harbour locus. Furthermore, an innovative type of anti-CD19 CAR-T cell with PD1 integration was developed and showed superior ability to eradicate tumour cells in xenograft models. In adoptive therapy for relapsed/refractory aggressive B cell non-Hodgkin lymphoma (ClinicalTrials.gov, NCT04213469 ), we observed a high rate (87.5%) of complete remission and durable responses without serious adverse events in eight patients. Notably, these enhanced CAR-T cells were effective even at a low infusion dose and with a low percentage of CAR+ cells. Single-cell analysis showed that the electroporation method resulted in a high percentage of memory T cells in infusion products, and PD1 interference enhanced anti-tumour immune functions, further validating the advantages of non-viral, PD1-integrated CAR-T cells. Collectively, our results demonstrate the high safety and efficacy of non-viral, gene-specific integrated CAR-T cells, thus providing an innovative technology for CAR-T cell therapy.
© 2022. The Author(s).
Conflict of interest statement
This study was partially supported by BRL Medicine, Inc. Patent applications related to this manuscript have been submitted (J.Z., J.Y., Y.T., B.D., Dali Li, M.L., Z.X. ‘sgRNA guiding
Figures







Comment in
-
A "CRISPR" non-viral manufacturing approach for CAR T cell therapies.Mol Ther. 2022 Nov 2;30(11):3338-3340. doi: 10.1016/j.ymthe.2022.09.022. Epub 2022 Oct 20. Mol Ther. 2022. PMID: 36265492 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

