Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression
- PMID: 36045402
- PMCID: PMC9429363
- DOI: 10.1186/s40168-022-01337-5
Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression
Abstract
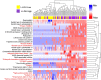
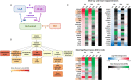
Background: Women with a cervicovaginal microbiota dominated by Lactobacillus spp. are at reduced risk of acquiring sexually transmitted infections including HIV, but the biological mechanisms involved remain poorly defined. Here, we performed metaproteomics on vaginal swab samples from young South African women (n = 113) and transcriptomics analysis of cervicovaginal epithelial cell cultures to examine the ability of lactic acid, a metabolite produced by cervicovaginal lactobacilli, to modulate genital epithelial barrier function.
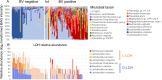
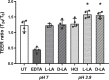
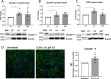
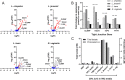
Results: Compared to women with Lactobacillus-depleted microbiota, women dominated by vaginal lactobacilli exhibit higher abundance of bacterial lactate dehydrogenase, a key enzyme responsible for lactic acid production, which is independently associated with an increased abundance of epithelial barrier proteins. Physiological concentrations of lactic acid enhance epithelial cell culture barrier integrity and increase intercellular junctional molecule expression.
Conclusions: These findings reveal a novel ability of vaginal lactic acid to enhance genital epithelial barrier integrity that may help prevent invasion by sexually transmitted pathogens. Video abstract.
Keywords: Epithelial cells; Female reproductive tract; HIV; Lactic acid; Lactobacilli; Metabolites; STIs; Tight junctions; Transcriptomics; Vaginal microbiome.
© 2022. The Author(s).
Conflict of interest statement
GT and ACH are coinventors on a patent application examining the immunomodulatory effects of L-lactic acid on cervicovaginal epithelial cells. The other authors declare that they have no competing interests.
Figures
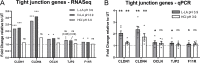
References
-
- Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, The Vaginal Microbiome Consortium. Jefferson KK, Buck GA, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading) 2014;160(Pt 10):2272–2282. doi: 10.1099/mic.0.081034-0. - DOI - PMC - PubMed
-
- Aldunate M, Srbinovski D, Hearps AC, Latham CF, Ramsland PA, Gugasyan R, et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

