Synthesis of doxorubicin-loaded peptosomes hybridized with gold nanorod for targeted drug delivery and CT imaging of metastatic breast cancer
- PMID: 36045404
- PMCID: PMC9429417
- DOI: 10.1186/s12951-022-01607-2
Synthesis of doxorubicin-loaded peptosomes hybridized with gold nanorod for targeted drug delivery and CT imaging of metastatic breast cancer
Abstract
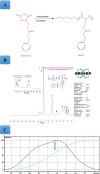
Background: Cancer nanomedicines based on synthetic polypeptides have attracted much attention due to their superior biocompatibility and biodegradability, stimuli responsive capability through secondary conformation change, adjustable functionalities for various cargos such as peptides, proteins, nucleic acids and small therapeutic molecules. Recently, a few nanoformulations based on polypeptides comprising NK105, NC6004, NK911, CT2103, have entered phase I-III clinical trials for advanced solid tumors therapy. In the current study, we prepared polypeptide-based vesicles called peptosome via self-assembly of amphiphilic polypeptide-based PEG-PBLG diblock copolymer.
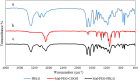
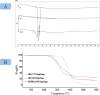
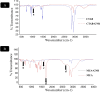
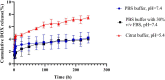
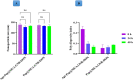
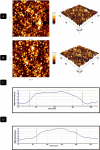
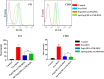
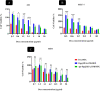
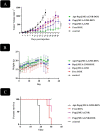
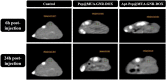
Results: In this regard, poly(γ-benzyl L-glutamate (PBLG) was synthesized via ring opening polymerization (ROP) of γ-benzyl L-glutamate-N-carboxyanhydride (BLG-NCA) using N-hexylamine as initiator. Then amine-terminated PBLG was covalently conjugated to heterofuctional maleimide PEG-carboxylic acid or methyl-PEG-carboxylic acid. The PEG-PBLG peptosomes were prepared through double emulsion method for the co-delivery of doxorubicin.HCl and gold nanorods as hydrophilic and hydrophobic agents in interior compartment and membrane of peptosomes, respectively (Pep@MUA.GNR-DOX) that DOX encapsulation efficiency and loading capacity were determined 42 ± 3.6 and 1.68 ± 3.6. Then, theranostic peptosomes were decorated with thiol-functionalized EpCAM aptamer throught thiol-maleimide reaction producing Apt-Pep@MUA.GNR-DOX for targeted delivery. The non-targeted and targeted peptosomes showed 165.5 ± 1.1 and 185 ± 4.7 nm diameters, respectively while providing sustained, controlled release of DOX. Furthermore, non-targeted and targeted peptosomes showed considerable serum stability. In vitro study on MCF-7 and 4T1 cells showed significantly higher cytotoxicity for Apt-Pep@MUA.GNR-DOX in comparison with Pep@MUA.GNR-DOX while both system did not show any difference in cytotoxicity against CHO cell line. Furthermore, Apt-Pep@MUA.GNR-DOX illustrated higher cellular uptake toward EpCAM-overexpressing 4T1 cells compared to Pep@MUA.GNR-DOX. In preclinical stage, therapeutic and diagnostic capability of the prepared Pep@MUA.GNR-DOX and Apt-Pep@MUA.GNR-DOX were investigated implementing subcutaneous 4T1 tumor model in BALB/c mice. The obtained data indicated highest therapeutic index for Apt-Pep@MUA.GNR-DOX compared to Pep@MUA.GNR-DOX and free DOX. Moreover, the prepared system showed capability of CT imaging of tumor tissue in 4T1 tumorized mice through tumor accumulation even 24 h post-administration.
Conclusion: In this regard, the synthesized theranostic peptosomes offer innovative hybrid multipurpose platform for fighting against breast cancer.
Keywords: Breast cancer; Doxorubicin; Gold nanorod; Peptosome; Theranostics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Targeted poly(L-glutamic acid)-based hybrid peptosomes co-loaded with doxorubicin and USPIONs as a theranostic platform for metastatic breast cancer.Nanomedicine. 2023 Feb;48:102645. doi: 10.1016/j.nano.2022.102645. Epub 2022 Dec 20. Nanomedicine. 2023. PMID: 36549556
-
Multifunctional PEG-b-polypeptide-decorated gold nanorod for targeted combined chemo-photothermal therapy of breast cancer.Colloids Surf B Biointerfaces. 2019 Sep 1;181:602-611. doi: 10.1016/j.colsurfb.2019.05.025. Epub 2019 May 12. Colloids Surf B Biointerfaces. 2019. PMID: 31202131
-
Nucleolin-targeted doxorubicin and ICG co-loaded theranostic lipopolymersome for photothermal-chemotherapy of melanoma in vitro and in vivo.Eur J Pharm Biopharm. 2024 Sep;202:114411. doi: 10.1016/j.ejpb.2024.114411. Epub 2024 Jul 14. Eur J Pharm Biopharm. 2024. PMID: 39009192
-
Thermally cross-linked superparamagnetic iron oxide nanoparticle-A10 RNA aptamer-doxorubicin conjugate.2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Aug 29 [updated 2008 Oct 8]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641568 Free Books & Documents. Review.
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
Cited by
-
Advances in peptide-based drug delivery systems.Heliyon. 2024 Feb 7;10(4):e26009. doi: 10.1016/j.heliyon.2024.e26009. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38404797 Free PMC article. Review.
-
Nanomaterial-based CT contrast agents and their applications in image-guided therapy.Theranostics. 2023 Jan 1;13(2):483-509. doi: 10.7150/thno.79625. eCollection 2023. Theranostics. 2023. PMID: 36632234 Free PMC article. Review.
-
Tackling breast cancer with gold nanoparticles: twinning synthesis and particle engineering with efficacy.Nanoscale Adv. 2024 Apr 17;6(11):2766-2812. doi: 10.1039/d3na00988b. eCollection 2024 May 29. Nanoscale Adv. 2024. PMID: 38817429 Free PMC article. Review.
-
Targeted co-delivery of FOXM1 aptamer and DOX by nucleolin aptamer-functionalized pH-responsive biocompatible nanodelivery system to enhance therapeutic efficacy against breast cancer: in vitro and in vivo.Drug Deliv Transl Res. 2024 Jun;14(6):1535-1550. doi: 10.1007/s13346-023-01495-5. Epub 2023 Dec 31. Drug Deliv Transl Res. 2024. PMID: 38161196
-
Physicochemical characterization and potential cancer therapy applications of hydrogel beads loaded with doxorubicin and GaOOH nanoparticles.Sci Rep. 2024 Sep 6;14(1):20822. doi: 10.1038/s41598-024-67709-z. Sci Rep. 2024. PMID: 39242631 Free PMC article.
References
-
- Mansur AAP, et al. Supramolecular magnetonanohybrids for multimodal targeted therapy of triple-negative breast cancer cells. J Mater Chem B. 2020;8(32):7166–7188. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Dhankhar R, Vyas SP, Jain AK, Arora S, Rath G, Goyal AK. Advances in novel drug delivery strategies for breast cancer therapy. Artif Cells, Blood Substitutes, Biotechnol. 2010;38(5):230–249. - PubMed
-
- Tacar O, Sriamornsak P, C. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

