Distinct volumetric features of cerebrospinal fluid distribution in idiopathic normal-pressure hydrocephalus and Alzheimer's disease
- PMID: 36045420
- PMCID: PMC9434899
- DOI: 10.1186/s12987-022-00362-8
Distinct volumetric features of cerebrospinal fluid distribution in idiopathic normal-pressure hydrocephalus and Alzheimer's disease
Abstract
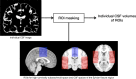
Objective: The aims of the study were to measure the cerebrospinal fluid (CSF) volumes in the lateral ventricle, high-convexity subarachnoid space, and Sylvian fissure region in patients with idiopathic normal-pressure hydrocephalus (INPH) and Alzheimer's disease (AD), and to evaluate differences in these volumes between INPH and AD groups and healthy controls.
Methods: Forty-nine INPH patients, 59 AD patients, and 26 healthy controls were imaged with automated three-dimensional volumetric MRI.
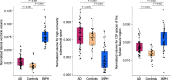
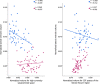
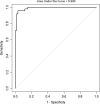
Results: INPH patients had larger lateral ventricles and CSF spaces of the Sylvian fissure region and smaller high-convexity subarachnoid spaces than other groups, and AD patients had larger lateral ventricles and CSF spaces of the Sylvian fissure region than the control group. The INPH group showed a negative correlation between lateral ventricle and high-convexity subarachnoid space volumes, while the AD group showed a positive correlation between lateral ventricle volume and volume for CSF spaces of the Sylvian fissure region. The ratio of lateral ventricle to high-convexity subarachnoid space volumes yielded an area under the curve of 0.990, differentiating INPH from AD.
Conclusions: Associations between CSF volumes suggest that there might be different mechanisms between INPH and AD to explain their respective lateral ventricular dilations. The ratio of lateral ventricle to high-convexity subarachnoid space volumes distinguishes INPH from AD with good diagnostic sensitivity and specificity. We propose to refer to this ratio as the VOSS (ventricle over subarachnoid space) index.
Keywords: Alzheimer’s disease; Cerebrospinal fluid space; Idiopathic normal-pressure hydrocephalus; Magnetic resonance imaging.
© 2022. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures
Similar articles
-
Hyperdynamic CSF motion profiles found in idiopathic normal pressure hydrocephalus and Alzheimer's disease assessed by fluid mechanics derived from magnetic resonance images.Fluids Barriers CNS. 2017 Oct 18;14(1):29. doi: 10.1186/s12987-017-0077-y. Fluids Barriers CNS. 2017. PMID: 29047355 Free PMC article.
-
Comparison of CSF Distribution between Idiopathic Normal Pressure Hydrocephalus and Alzheimer Disease.AJNR Am J Neuroradiol. 2016 Jul;37(7):1249-55. doi: 10.3174/ajnr.A4695. Epub 2016 Feb 25. AJNR Am J Neuroradiol. 2016. PMID: 26915568 Free PMC article.
-
Detection of changes in cerebrospinal fluid space in idiopathic normal pressure hydrocephalus using voxel-based morphometry.Neuroradiology. 2010 May;52(5):381-6. doi: 10.1007/s00234-009-0610-z. Epub 2009 Oct 22. Neuroradiology. 2010. PMID: 19847409
-
[Hypothesis of cerebrospinal fluid formation and absorption: from the clinical viewpoint of idiopathic normal pressure hydrocephalus].Rinsho Shinkeigaku. 2014;54(12):1190-2. doi: 10.5692/clinicalneurol.54.1190. Rinsho Shinkeigaku. 2014. PMID: 25672742 Review. Japanese.
-
Brain MRI as a predictor of CSF tap test response in patients with idiopathic normal pressure hydrocephalus.J Neurol. 2010 Oct;257(10):1675-81. doi: 10.1007/s00415-010-5602-8. Epub 2010 May 29. J Neurol. 2010. PMID: 20512347 Review.
Cited by
-
Individual-level cortical morphological network analysis in idiopathic normal pressure hydrocephalus: diagnostic and prognostic insights.Fluids Barriers CNS. 2025 May 6;22(1):43. doi: 10.1186/s12987-025-00653-w. Fluids Barriers CNS. 2025. PMID: 40329395 Free PMC article.
-
The urotensin II receptor triggers an early meningeal response and a delayed macrophage-dependent vasospasm after subarachnoid hemorrhage in male mice.Nat Commun. 2024 Sep 29;15(1):8430. doi: 10.1038/s41467-024-52654-2. Nat Commun. 2024. PMID: 39341842 Free PMC article.
-
CT-based volumetric measures obtained through deep learning: Association with biomarkers of neurodegeneration.Alzheimers Dement. 2024 Jan;20(1):629-640. doi: 10.1002/alz.13445. Epub 2023 Sep 28. Alzheimers Dement. 2024. PMID: 37767905 Free PMC article.
-
Methodological challenges of measuring brain volumes and cortical thickness in idiopathic normal pressure hydrocephalus with a surface-based approach.Front Neurosci. 2024 Jul 19;18:1366029. doi: 10.3389/fnins.2024.1366029. eCollection 2024. Front Neurosci. 2024. PMID: 39099637 Free PMC article.
-
Association between idiopathic normal pressure hydrocephalus and Alzheimer's disease: a bidirectional Mendelian randomization study.Sci Rep. 2024 Sep 30;14(1):22744. doi: 10.1038/s41598-024-72559-w. Sci Rep. 2024. PMID: 39349954 Free PMC article.
References
-
- Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4–16. - PubMed
-
- Benedetto N, Gambacciani C, Aquila F, Di Carlo DT, Morganti R, Perrini P. A new quantitative method to assess disproportionately enlarged subarachnoid space (DESH) in patients with possible idiopathic normal pressure hydrocephalus: the SILVER index. Clin Neurol Neurosurg. 2017;158:27–32. doi: 10.1016/j.clineuro.2017.04.015. - DOI - PubMed
MeSH terms
Grants and funding
- NRF-2019R1I1A3A01053926/Basic Science Research Program through the National Research Foundation of Korea (NRF)
- NRF-2019R1D1A3A03103893/Basic Science Research Program through the National Research Foundation of Korea (NRF)
- NRF-2021R1I1A3058731/Basic Science Research Program through the National Research Foundation of Korea (NRF)
LinkOut - more resources
Full Text Sources
Medical

