Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia
- PMID: 36045450
- PMCID: PMC9429339
- DOI: 10.1186/s13195-022-01042-3
Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia
Abstract
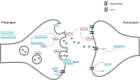
Background: Approximately a third of frontotemporal dementia (FTD) is genetic with mutations in three genes accounting for most of the inheritance: C9orf72, GRN, and MAPT. Impaired synaptic health is a common mechanism in all three genetic variants, so developing fluid biomarkers of this process could be useful as a readout of cellular dysfunction within therapeutic trials.
Methods: A total of 193 cerebrospinal fluid (CSF) samples from the GENetic FTD Initiative including 77 presymptomatic (31 C9orf72, 23 GRN, 23 MAPT) and 55 symptomatic (26 C9orf72, 17 GRN, 12 MAPT) mutation carriers as well as 61 mutation-negative controls were measured using a microflow LC PRM-MS set-up targeting 15 synaptic proteins: AP-2 complex subunit beta, complexin-2, beta-synuclein, gamma-synuclein, 14-3-3 proteins (eta, epsilon, zeta/delta), neurogranin, Rab GDP dissociation inhibitor alpha (Rab GDI alpha), syntaxin-1B, syntaxin-7, phosphatidylethanolamine-binding protein 1 (PEBP-1), neuronal pentraxin receptor (NPTXR), neuronal pentraxin 1 (NPTX1), and neuronal pentraxin 2 (NPTX2). Mutation carrier groups were compared to each other and to controls using a bootstrapped linear regression model, adjusting for age and sex.
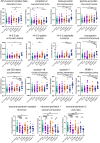
Results: CSF levels of eight proteins were increased only in symptomatic MAPT mutation carriers (compared with controls) and not in symptomatic C9orf72 or GRN mutation carriers: beta-synuclein, gamma-synuclein, 14-3-3-eta, neurogranin, Rab GDI alpha, syntaxin-1B, syntaxin-7, and PEBP-1, with three other proteins increased in MAPT mutation carriers compared with the other genetic groups (AP-2 complex subunit beta, complexin-2, and 14-3-3 zeta/delta). In contrast, CSF NPTX1 and NPTX2 levels were affected in all three genetic groups (decreased compared with controls), with NPTXR concentrations being affected in C9orf72 and GRN mutation carriers only (decreased compared with controls). No changes were seen in the CSF levels of these proteins in presymptomatic mutation carriers. Concentrations of the neuronal pentraxins were correlated with brain volumes in the presymptomatic period for the C9orf72 and GRN groups, suggesting that they become abnormal in proximity to symptom onset.
Conclusions: Differential synaptic impairment is seen in the genetic forms of FTD, with abnormalities in multiple measures in those with MAPT mutations, but only changes in neuronal pentraxins within the GRN and C9orf72 mutation groups. Such markers may be useful in future trials as measures of synaptic dysfunction, but further work is needed to understand how these markers change throughout the course of the disease.
Keywords: Biomarkers; Frontotemporal dementia; Synaptic dysfunction.
© 2022. The Author(s).
Conflict of interest statement
KB has served as a consultant, at advisory boards or at data monitoring committees, for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineer, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. JDR has served as a consultant or on an advisory board for Alector, Prevail Therapeutics, Denali, Arkuda Therapeutics, Takeda, UCB, Wave Life Sciences, and Novartis. The other authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

