A Phase 2 open label study of ledipasvir/sofosbuvir for 12 weeks in subjects with hepatitis B virus infection
- PMID: 36045503
- PMCID: PMC10087219
- DOI: 10.1002/jmv.28105
A Phase 2 open label study of ledipasvir/sofosbuvir for 12 weeks in subjects with hepatitis B virus infection
Abstract
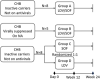
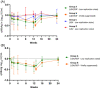
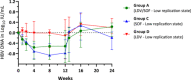
Retrospective data showed that when we administered ledipasvir/sofosbuvir (LDV/SOF) to patients with hepatitis B and C coinfection, there was a modest reduction in hepatitis B surface antigen (HBsAg). Therefore, we hypothesize that similar HBsAg reduction can be seen in hepatitis B virus (HBV) monoinfected subjects. Primary and secondary efficacy endpoints are the decline in HBsAg and HBV DNA at Week 12 from baseline, respectively. We conducted an open-label Phase 2 pilot study to evaluate the safety, tolerability, and antiviral activity of LDV and/or SOF for HBV. Eligible subjects were either suppressed on antivirals (Group B) or inactive chronic HBV (Group A, C, D). Group A and B received LDV/SOF. Group C and D received SOF 400 mg and LDV 90 mg, respectively. All subjects completed the study, and all related adverse events (AEs) were mild. No discontinuations due to AEs or hepatitis flare occurred. At Week 12, HBsAg decline (log10 IU/ml) was similar between Group A (0.399) and B (0.400), less in Group C (0.207), and none in Group D, and there was HBV DNA decline in the inactive chronic HBV groups. LDV and SOF are safe and well tolerated when given to chronic hepatitis B subjects and have modest antiviral activity, particularly when given in combination.
Keywords: HBV DNA; HBV infection; antiviral effect; chronic hepatitis B; hepatitis B surface antigen; ledipasvir; nucleos(t)ide analogues; quantitative HBV surface antigen; sofosbuvir; viral hepatitis B.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Efficacy of Ledipasvir and Sofosbuvir Treatment of HCV Infection in Patients Coinfected With HBV.Gastroenterology. 2018 Mar;154(4):989-997. doi: 10.1053/j.gastro.2017.11.011. Epub 2017 Nov 22. Gastroenterology. 2018. PMID: 29174546 Clinical Trial.
-
Ledipasvir/Sofosbuvir for Patients Coinfected With Chronic Hepatitis C and Hepatitis B in Taiwan: Follow-up at 108 Weeks Posttreatment.Clin Infect Dis. 2022 Aug 31;75(3):453-459. doi: 10.1093/cid/ciab971. Clin Infect Dis. 2022. PMID: 34864948 Free PMC article.
-
Multicenter Experience using Ledipasvir/Sofosbuvir ± RBV to Treat HCV GT 1 Relapsers after Simeprevir and Sofosbuvir Treatment.Ann Hepatol. 2018 Aug 24;17(5):815-821. doi: 10.5604/01.3001.0012.3142. Ann Hepatol. 2018. PMID: 30145562
-
Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature.BMC Infect Dis. 2017 Mar 1;17(1):182. doi: 10.1186/s12879-017-2287-y. BMC Infect Dis. 2017. PMID: 28249574 Free PMC article. Review.
-
Clinical utility of quantitative HBsAg in natural history and nucleos(t)ide analogue treatment of chronic hepatitis B: new trick of old dog.J Gastroenterol. 2013 Jan;48(1):13-21. doi: 10.1007/s00535-012-0668-y. Epub 2012 Oct 24. J Gastroenterol. 2013. PMID: 23090000 Free PMC article. Review.
Cited by
-
Nanoparticle-based therapeutic strategies for chronic liver diseases: Advances and insights.Liver Res. 2025 Apr 12;9(2):104-117. doi: 10.1016/j.livres.2025.04.002. eCollection 2025 Jun. Liver Res. 2025. PMID: 40620504 Free PMC article. Review.
-
Hepatitis B: Model Systems and Therapeutic Approaches.J Immunol Res. 2024 May 7;2024:4722047. doi: 10.1155/2024/4722047. eCollection 2024. J Immunol Res. 2024. PMID: 38745751 Free PMC article. Review.
References
-
- World Health Organization . Hepatitis B. Published June 24, 2022. Accessed September 6, 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
-
- Lok ASF, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661‐662. - PubMed
-
- European Association for the Study of the Liver . EASL clinical practice guidelines. management of chronic hepatitis B. Gastroenterol Clin Biol. 2009;33(6‐7):539‐554. - PubMed
-
- Yuen MF, Wong DK, Fung J, et al. HBsAg seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192‐1199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

