FOXP3 expression diversifies the metabolic capacity and enhances the efficacy of CD8 T cells in adoptive immunotherapy of melanoma
- PMID: 36045586
- PMCID: PMC9840123
- DOI: 10.1016/j.ymthe.2022.08.017
FOXP3 expression diversifies the metabolic capacity and enhances the efficacy of CD8 T cells in adoptive immunotherapy of melanoma
Abstract
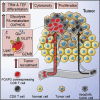
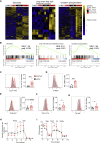
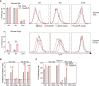
Regulatory T cells overwhelm conventional T cells in the tumor microenvironment (TME) thanks to a FOXP3-driven metabolic program that allows them to engage different metabolic pathways. Using a melanoma model of adoptive T cell therapy (ACT), we show that FOXP3 overexpression in mature CD8 T cells improved their antitumor efficacy, favoring their tumor recruitment, proliferation, and cytotoxicity. FOXP3-overexpressing (Foxp3UP) CD8 T cells exhibited features of tissue-resident memory-like and effector T cells, but not suppressor activity. Transcriptomic analysis of tumor-infiltrating Foxp3UP CD8 T cells showed positive enrichment in a wide variety of metabolic pathways, such as glycolysis, fatty acid (FA) metabolism, and oxidative phosphorylation (OXPHOS). Intratumoral Foxp3UP CD8 T cells exhibited an enhanced capacity for glucose and FA uptake as well as accumulation of intracellular lipids. Interestingly, Foxp3UP CD8 T cells compensated for the loss of mitochondrial respiration-driven ATP production by activating aerobic glycolysis. Moreover, in limiting nutrient conditions these cells engaged FA oxidation to drive OXPHOS for their energy demands. Importantly, their ability to couple glycolysis and OXPHOS allowed them to sustain proliferation under glucose restriction. Our findings demonstrate a hitherto unknown role for FOXP3 in the adaptation of CD8 T cells to TME that may enhance their efficacy in ACT.
Keywords: CD8 T cell response; FOXP3; T cell metabolism; T cell-based cancer immunotherapy.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
TCR-induced FOXP3 expression by CD8+ T cells impairs their anti-tumor activity.Cancer Lett. 2022 Mar 1;528:45-58. doi: 10.1016/j.canlet.2021.12.030. Epub 2021 Dec 29. Cancer Lett. 2022. PMID: 34973390
-
Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function.J Clin Invest. 2013 Oct;123(10):4479-88. doi: 10.1172/JCI69589. Epub 2013 Sep 16. J Clin Invest. 2013. PMID: 24091329 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
Metabolic Reprogramming of Glycolysis, Lipids, and Amino Acids in Tumors: Impact on CD8+ T Cell Function and Targeted Therapeutic Strategies.FASEB J. 2025 Apr 30;39(8):e70520. doi: 10.1096/fj.202403019R. FASEB J. 2025. PMID: 40249661 Review.
-
Tissue-resident memory CD8+ T cells in cancer immunology and immunotherapy.Pharmacol Res. 2020 Sep;159:104876. doi: 10.1016/j.phrs.2020.104876. Epub 2020 May 16. Pharmacol Res. 2020. PMID: 32422340 Review.
Cited by
-
IFI27 enhances bladder cancer immunotherapy response by modulating regulatory T cell enrichment.J Cancer. 2024 Oct 28;15(20):6616-6630. doi: 10.7150/jca.99014. eCollection 2024. J Cancer. 2024. PMID: 39668835 Free PMC article.
-
Tissue-resident memory T cells in diseases and therapeutic strategies.MedComm (2020). 2025 Jan 12;6(1):e70053. doi: 10.1002/mco2.70053. eCollection 2025 Jan. MedComm (2020). 2025. PMID: 39802636 Free PMC article. Review.
-
Specific Polo-Like Kinase 1 Expression in Nodular Lymphocyte-Predominant Hodgkin Lymphoma Suggests an Intact Immune Surveillance Program.Am J Pathol. 2024 Jan;194(1):165-178. doi: 10.1016/j.ajpath.2023.10.008. Epub 2023 Nov 3. Am J Pathol. 2024. PMID: 37923249 Free PMC article.
-
Lipid metabolism in tumor-infiltrating T cells: mechanisms and applications.Life Metab. 2022 Dec 15;1(3):211-223. doi: 10.1093/lifemeta/loac038. eCollection 2022 Dec. Life Metab. 2022. PMID: 39872079 Free PMC article. Review.
-
Lipid metabolism in tumor-infiltrating regulatory T cells: perspective to precision immunotherapy.Biomark Res. 2024 Apr 22;12(1):41. doi: 10.1186/s40364-024-00588-8. Biomark Res. 2024. PMID: 38644503 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

