Hypoxia and hypoxia-inducible factor signals regulate the development, metabolism, and function of B cells
- PMID: 36045669
- PMCID: PMC9421003
- DOI: 10.3389/fimmu.2022.967576
Hypoxia and hypoxia-inducible factor signals regulate the development, metabolism, and function of B cells
Abstract
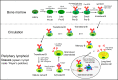
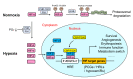
Hypoxia is a common hallmark of healthy tissues in physiological states or chronically inflamed tissues in pathological states. Mammalian cells sense and adapt to hypoxia mainly through hypoxia-inducible factor (HIF) signaling. Many studies have shown that hypoxia and HIF signaling play an important regulatory role in development and function of innate immune cells and T cells, but their role in B cell biology is still controversial. B cells experience a complex life cycle (including hematopoietic stem cells, pro-B cells, pre-B cells, immature B cells, mature naïve B cells, activated B cells, plasma cells, and memory B cells), and the partial pressure of oxygen (PO2) in the corresponding developmental niche of stage-specific B cells is highly dynamic, which suggests that hypoxia and HIF signaling may play an indispensable role in B cell biology. Based on the fact that hypoxia niches exist in the B cell life cycle, this review focuses on recent discoveries about how hypoxia and HIF signaling regulate the development, metabolism, and function of B cells, to facilitate a deep understanding of the role of hypoxia in B cell-mediated adaptive immunity and to provide novel strategies for vaccine adjuvant research and the treatment of immunity-related or infectious diseases.
Keywords: B cell biology; development; function; hypoxia; hypoxia-inducible factor signaling; metabolism.
Copyright © 2022 Zhang, Wu, Ma, Long, Sun, Li and Ge.
Conflict of interest statement
Authors JZ and LG were employed by the company Chongqing Camab Biotech Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Hypoxia and B cells.Exp Cell Res. 2017 Jul 15;356(2):197-203. doi: 10.1016/j.yexcr.2017.03.019. Epub 2017 Mar 12. Exp Cell Res. 2017. PMID: 28300562 Review.
-
Hypoxia and the Hypoxia-Inducible Factors in Lymphocyte Differentiation and Function.Adv Exp Med Biol. 2024;1459:115-141. doi: 10.1007/978-3-031-62731-6_6. Adv Exp Med Biol. 2024. PMID: 39017842 Review.
-
Hypoxia-inducible factors in T lymphocyte differentiation and function. A Review in the Theme: Cellular Responses to Hypoxia.Am J Physiol Cell Physiol. 2015 Nov 1;309(9):C580-9. doi: 10.1152/ajpcell.00204.2015. Epub 2015 Sep 9. Am J Physiol Cell Physiol. 2015. PMID: 26354751 Free PMC article. Review.
-
Hypoxia-inducible factors regulate T cell metabolism and function.Mol Immunol. 2015 Dec;68(2 Pt C):527-35. doi: 10.1016/j.molimm.2015.08.004. Epub 2015 Aug 19. Mol Immunol. 2015. PMID: 26298577 Free PMC article. Review.
-
Oxygen sensing strategies in mammals and bacteria.J Inorg Biochem. 2014 Apr;133:63-72. doi: 10.1016/j.jinorgbio.2013.12.010. Epub 2014 Jan 3. J Inorg Biochem. 2014. PMID: 24468676 Free PMC article. Review.
Cited by
-
Microbiota-dependent indole production stimulates the development of collagen-induced arthritis in mice.J Clin Invest. 2023 Dec 19;134(4):e167671. doi: 10.1172/JCI167671. J Clin Invest. 2023. PMID: 38113112 Free PMC article.
-
Changes in immune cell populations during acclimatization to high altitude.Physiol Rep. 2024 Nov;12(22):e70024. doi: 10.14814/phy2.70024. Physiol Rep. 2024. PMID: 39551933 Free PMC article.
-
Hypoxic tumor microenvironment: Destroyer of natural killer cell function.Chin J Cancer Res. 2024 Apr 30;36(2):138-150. doi: 10.21147/j.issn.1000-9604.2024.02.04. Chin J Cancer Res. 2024. PMID: 38751439 Free PMC article.
-
Immune Checkpoints in B Cells: Unlocking New Potentials in Cancer Treatment.Adv Sci (Weinh). 2024 Dec;11(47):e2403423. doi: 10.1002/advs.202403423. Epub 2024 Nov 7. Adv Sci (Weinh). 2024. PMID: 39509319 Free PMC article. Review.
-
Roles of hypoxia inducible factors in viral infection: Are they a potential therapeutic target?Virulence. 2025 Dec;16(1):2546680. doi: 10.1080/21505594.2025.2546680. Epub 2025 Aug 13. Virulence. 2025. PMID: 40802588 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

