Cigarette smoke-induced gasdermin D activation in bronchoalveolar macrophages and bronchial epithelial cells dependently on NLRP3
- PMID: 36045672
- PMCID: PMC9421433
- DOI: 10.3389/fimmu.2022.918507
Cigarette smoke-induced gasdermin D activation in bronchoalveolar macrophages and bronchial epithelial cells dependently on NLRP3
Abstract
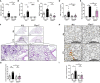
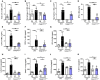
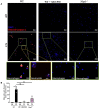
Chronic pulmonary inflammation and chronic obstructive pulmonary disease (COPD) are major health issues largely due to air pollution and cigarette smoke (CS) exposure. The role of the innate receptor NLRP3 (nucleotide-binding domain and leucine-rich repeat containing protein 3) orchestrating inflammation through formation of an inflammasome complex in CS-induced inflammation or COPD remains controversial. Using acute and subchronic CS exposure models, we found that Nlrp3-deficient mice or wild-type mice treated with the NLRP3 inhibitor MCC950 presented an important reduction of inflammatory cells recruited into the bronchoalveolar space and of pulmonary inflammation with decreased chemokines and cytokines production, in particular IL-1β demonstrating the key role of NLRP3. Furthermore, mice deficient for Caspase-1/Caspase-11 presented also decreased inflammation parameters, suggesting a role for the NLRP3 inflammasome. Importantly we showed that acute CS-exposure promotes NLRP3-dependent cleavage of gasdermin D in macrophages present in the bronchoalveolar space and in bronchial airway epithelial cells. Finally, Gsdmd-deficiency reduced acute CS-induced lung and bronchoalveolar space inflammation and IL-1β secretion. Thus, we demonstrated in our model that NLRP3 and gasdermin D are key players in CS-induced pulmonary inflammation and IL-1β release potentially through gasdermin D forming-pore and/or pyroptoctic cell death.
Keywords: NLRP3 inflammasome; cigarette smoke; gasdermin D; lung; mice.
Copyright © 2022 Huot-Marchand, Nascimento, Culerier, Bourenane, Savigny, Panek, Serdjebi, Le Bert, Quesniaux, Ryffel, Broz, Riteau, Gombault and Couillin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

