A protocol for a randomized controlled trial comparing Sleepwell, EMPOWER, and treatment-as-usual for benzodiazepine receptor agonist discontinuation in older adults: the your answers when needing sleep in New Brunswick (YAWNS NB) study
- PMID: 36045710
- PMCID: PMC9420952
- DOI: 10.1016/j.rcsop.2022.100164
A protocol for a randomized controlled trial comparing Sleepwell, EMPOWER, and treatment-as-usual for benzodiazepine receptor agonist discontinuation in older adults: the your answers when needing sleep in New Brunswick (YAWNS NB) study
Abstract
Background: Chronic benzodiazepine receptor agonist (BZRA) use among older adults is a public health concern given cognitive and physical risks. One in four older adults in New Brunswick, Canada, is a long-term user of BZRAs. Previous studies using a direct-to-patient approach as the primary intervention target have shown promise in reducing BZRA use. The Your Answers When Needing Sleep in New Brunswick (YAWNS NB) study aims to reduce the long-term use of BZRAs in older adults and increase the use of cognitive behavioural therapy for insomnia (CBTi), which is the recommended first line treatment.
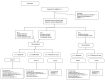
Methods: The trial (ClinicalTrials.gov registration NCT04406103) is a three arm, open-label, parallel randomized controlled trial in NB, Canada. Eligible participants 65 years and older using BZRAs long-term will be randomly allocated to: the Eliminating Medications through Patient Ownership of End Results (EMPOWER) information package group; the Sleepwell information package group; or treatment-as-usual (TAU). Information packages will be mailed via Canada Post. The primary outcome of BZRA discontinuation at 6 months will be compared across groups. Secondary outcomes include participants with ≥25% BZRA dose reduction, and switching to newly prescribed alternate sedative-hypnotics. Several exploratory outcomes will also be examined.
Discussion: Targeting participants with information packages informing them of appropriate use, dangers, and approaches to reducing BZRA use and increasing CBTi use may be beneficial in a region of Canada with the highest rate of chronic BZRA use in older adults. Comparing information packages and TAU will provide insights into the effectiveness of direct-to-patient interventions for BZRA reduction.
Keywords: Benzodiazepine receptor agonists (BZRAs); Benzodiazepines; Chronic insomnia; Cognitive-behavioural therapy for insomnia (CBTi); EMPOWER; Sedative-hypnotic; Sleepwell; Z-drugs.
© 2022 The Authors.
Conflict of interest statement
None.
Figures
References
-
- Government of Canada SC Population Estimates, Quarterly. 2022. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 Published December 16, 2021. Accessed April 25.
-
- Canadian Institute for Health Information The Use of Selected Psychotropic Drugs Among Seniors on Public Drug Programs in Canada, 2001 to 2010. 2022. https://secure.cihi.ca/free_products/psychotropic_AIB_2012_en.pdf Published online March 2012. Accessed April 25.
-
- Canadian Institute for Health Information Pan-Canadian Trends in the Prescribing of Opioids and Benzodiazepines, 2012 to 2017. 2022. https://www.cihi.ca/sites/default/files/document/opioid-prescribing-june... Published online 2018. Accessed April 25.
-
- Canadian Deprescribing Network 2017 Annual Report. 2022. https://www.deprescribingnetwork.ca/reports Accessed April 25, 2022.
-
- Canadian Institute of Health Information . 2018. Drug Use among Seniors in Canada, 2016; pp. 1–63.www.cihi.ca
Associated data
LinkOut - more resources
Full Text Sources
Medical