CLI nical P rofile and S ide E ffects of chronic use of oral A miodarone in cardiology outpatients department (CLIPSE-A Study)- A prospective observational study
- PMID: 36045807
- PMCID: PMC9422214
- DOI: 10.1016/j.amsu.2022.104167
CLI nical P rofile and S ide E ffects of chronic use of oral A miodarone in cardiology outpatients department (CLIPSE-A Study)- A prospective observational study
Abstract
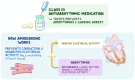
Background: Amiodarone belongs to Class-III anti-arrhythmic drugs. It is one of the most effective anti-arrhythmic drugs used to treat or prevent several types of arrhythmias including atrial fibrillation, atrial flutter, ventricular tachycardia, and wide complex tachycardia, but unfortunately carries a high toxicity profile. Also, side effects of amiodarone involving various organs can be life-threatening.
Materials & methods: This was an observational study carried out for six months i.e from April to September. The study included patients who are on amiodarone for greater than or equal to six months. The required data was collected in-person from the case sheets, treatment charts, and by interviewing the patients. The data for 67 patients was documented in suitable data collection form for analysis.
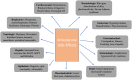
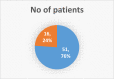
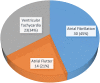
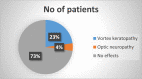
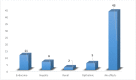
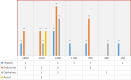
Results: From our study data, it was noted that amiodarone was used for 3 different indications-atrial fibrillation, atrial flutter, and ventricular tachycardia. Among 67 patients enrolled, 38 had no side-effects. Side-effects data in the rest grouped basing on the organ system affected: 9 patients had renal effects, 6 patients had ophthalmic effects, 4 patients had endocrine effects, and 5 patients had hepatic effects.
Conclusion: From our study, it is concluded that amiodarone is a safe and effective anti-arrhythmic drug at lower doses i.e. 200-1100 mg/week. When treated in lower doses of 1400-2800 mg/week, many side effects have been incident. Although these effects are mild and develop only after prolonged usage of the drug, it should be used judiciously.
Keywords: AF, Atrial fibrillation; AIH, Amiodarone induced hypothyroidism; AIT, Amiodarone induced thyrotoxicosis; AV, Atrioventricular; Amiodarone; Atrial fibrillation; Atrial flutter; BMI, Body mass index; CPR, Cardiopulmonary resuscitation; DEA, Des ethyl amiodarone; FDA, Food and drug administration; INR, International normalized ratio; PSVT, Paraoxysmal supraventricular tachycardia; Paroxysmal atrial fibrillation; SGOT, Serum glutamic-oxaloacetic transaminase; SGPT, Serum glutamic pyruvic transaminase; Side-effects; VF, Ventricular Fibrillation; VT, Ventricular tachycardia; Ventricular tachycardia.
© 2022 The Author(s).
Figures



References
-
- Swapna G., Pravallika B., Poojitha J. A review on drug-drug interaction studies on amiodarone and Levofloxacin. Res. J. Pharmacol. Pharmacodyn. 2019;11(4):147–152.
-
- Connolly S.J. Evidence-based analysis of Amiodarone efficacy and safety. Circulation. 1999 Nov 9;100(19):2025–2034. - PubMed
-
- Soar J., Perkins G.D., Maconochie I., Böttiger B.W., Deakin C.D., Sandroni C., Olasveengen T.M., Wyllie J., Greif R., Lockey A., Semeraro F. European Resuscitation Council Guidelines for Resuscitation: 2018 update–antiarrhythmic drugs for cardiac arrest. Resuscitation. 2019 Jan 1;134:99–103. - PubMed
-
- Yamreudeewong W., DeBisschop M., Martin L.G., Lower D.L. Potentially significant drug interactions of class III antiarrhythmic drugs. Drug Saf. 2003 May;26(6):421–438. - PubMed
-
- Kilborn M.J., Rathore S.S., Gersh B.J., Oetgen W.J., Solomon A.J. Amiodarone and mortality among elderly patients with acute myocardial infarction with atrial fibrillation. Am. Heart J. 2002 Dec 1;144(6):1095–1101. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

