Phosphate Frustration: Treatment Options to Complement Current Therapies
- PMID: 36045900
- PMCID: PMC9424003
- DOI: 10.1155/2022/9457440
Phosphate Frustration: Treatment Options to Complement Current Therapies
Abstract
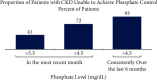
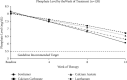
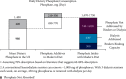
Hyperphosphatemia eventually develops in almost all patients with advanced chronic kidney disease and is associated with negative clinical outcomes. Thus, guidelines recommend targeting treatment to normal phosphate levels in patients with chronic kidney disease. Despite low phosphorus diets, clearance by dialysis, and phosphate binder use, many patients with chronic kidney disease on dialysis are unable to consistently achieve and maintain serum phosphate concentrations <5.5 mg/dL. A chart audit of patients on dialysis receiving phosphate binders showed that 74 to 86% were unable to consistently achieve serum phosphate ≤5.5 mg/dL over 6 months. Furthermore, although there is evidence that serum phosphate concentrations <4.5 mg/dL are associated with improved survival and cardiovascular outcomes, real-world phosphate control data suggest achieving and maintaining this goal for most patients would be extremely challenging, if not near impossible, using current therapies. As phosphate binders can only remove approximately 300 mg of the 2,500 mg or more daily dietary phosphate intake, therapeutic innovations are necessary to improve phosphate management. We present treatment options to complement current therapies including tenapanor, a novel sodium/hydrogen exchanger isoform 3 inhibitor that blocks the dominant paracellular phosphate absorption pathway and has been shown to reduce phosphate levels in several clinical trials.
Copyright © 2022 Pablo E. Pergola.
Conflict of interest statement
Dr. Pergola is a consultant for Ardelyx. He reports personal fees from Akebia Therapeutics, AstraZeneca, Bayer, Reata Pharmaceuticals, Gilead Sciences, Corvidia Therapeutics, FibroGen, Tricida, Ardelyx, Unicycive Therapeutic, and Otsuka Pharmaceuticals. For his performance as a PI, his employer Renal Associates, PA receives funding. He has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflicts with the subject matter or materials discussed in the manuscript apart from those disclosed. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Figures
Similar articles
-
A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY).J Am Soc Nephrol. 2021 Jun 1;32(6):1465-1473. doi: 10.1681/ASN.2020101398. Epub 2021 Mar 25. J Am Soc Nephrol. 2021. PMID: 33766811 Free PMC article. Clinical Trial.
-
Phosphate Absorption and Hyperphosphatemia Management in Kidney Disease: A Physiology-Based Review.Kidney Med. 2021 Aug 27;3(6):1057-1064. doi: 10.1016/j.xkme.2021.07.003. eCollection 2021 Nov-Dec. Kidney Med. 2021. PMID: 34939015 Free PMC article. Review.
-
Small Intestinal Phosphate Absorption: Novel Therapeutic Implications.Am J Nephrol. 2021;52(7):522-530. doi: 10.1159/000518110. Epub 2021 Aug 19. Am J Nephrol. 2021. PMID: 34515051 Review.
-
Tenapanor: A Phosphate Absorption Inhibitor for the Management of Hyperphosphatemia in Patients With Kidney Failure.J Ren Nutr. 2025 Jan;35(1):25-34. doi: 10.1053/j.jrn.2024.07.003. Epub 2024 Jul 9. J Ren Nutr. 2025. PMID: 38992521 Review.
-
Therapeutic Effects of Add-On Tenapanor for Hemodialysis Patients with Refractory Hyperphosphatemia.Am J Nephrol. 2021;52(6):496-506. doi: 10.1159/000516156. Epub 2021 Jun 7. Am J Nephrol. 2021. PMID: 34098559 Free PMC article. Clinical Trial.
Cited by
-
Pharmacological Nephroprotection in Non-Diabetic Chronic Kidney Disease-Clinical Practice Position Statement of the Polish Society of Nephrology.J Clin Med. 2023 Aug 9;12(16):5184. doi: 10.3390/jcm12165184. J Clin Med. 2023. PMID: 37629226 Free PMC article. Review.
-
Phosphate Control in Peritoneal Dialysis Patients: Issues, Solutions, and Open Questions.Nutrients. 2023 Jul 16;15(14):3161. doi: 10.3390/nu15143161. Nutrients. 2023. PMID: 37513579 Free PMC article. Review.
References
-
- Bansal V. K. Clinical Methods: The History, Physical, and Laboratory Examinations . 3. Boston, MA, USA: Butterworths; 1990. Serum inorganic phosphorus. - PubMed
-
- Chronic Kidney Disease in the United States . Atlanta, GA, USA: Centers for Disease Control and Prevention, Services UDoHaH; 2021.
-
- Phosphate binder use, DOPPS practice monitor. 2022. https://www.dopps.org/DPM-HD/Files/maxPBINDER_use_c_overallTAB.htm .
-
- Qadeer H. A., Bashir K. Physiology, Phosphate. StatPearls . Treasure Island, FL, USA: StatPearls Publishing; 2020. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

