Plankton food webs in the oligotrophic Gulf of Mexico spawning grounds of Atlantic bluefin tuna
- PMID: 36045950
- PMCID: PMC9424712
- DOI: 10.1093/plankt/fbab023
Plankton food webs in the oligotrophic Gulf of Mexico spawning grounds of Atlantic bluefin tuna
Abstract
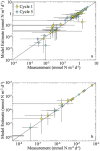
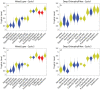
We used linear inverse ecosystem modeling techniques to assimilate data from extensive Lagrangian field experiments into a mass-balance constrained food web for the Gulf of Mexico open-ocean ecosystem. This region is highly oligotrophic, yet Atlantic bluefin tuna (ABT) travel long distances from feeding grounds in the North Atlantic to spawn there. Our results show extensive nutrient regeneration fueling primary productivity (mostly by cyanobacteria and other picophytoplankton) in the upper euphotic zone. The food web is dominated by the microbial loop (>70% of net primary productivity is respired by heterotrophic bacteria and protists that feed on them). By contrast, herbivorous food web pathways from phytoplankton to metazoan zooplankton process <10% of the net primary production in the mixed layer. Nevertheless, ABT larvae feed preferentially on podonid cladocerans and other suspension-feeding zooplankton, which in turn derive much of their nutrition from nano- and micro-phytoplankton (mixotrophic flagellates, and to a lesser extent, diatoms). This allows ABT larvae to maintain a comparatively low trophic level (~4.2 for preflexion and postflexion larvae), which increases trophic transfer from phytoplankton to larval fish.
Keywords: calanoid copepods; larval fish; marine food web; nitrogen cycle; plankton ecology.
© The Author(s) 2021. Published by Oxford University Press.
Figures





References
-
- Alemany, F., Quintanilla, L., Velez-Belchí, P., García, A., Cortés, D., Rodríguez, J. M., dePuelles, M. F., González-Pola, C.et al. (2010) Characterization of the spawning habitat of Atlantic bluefin tuna and related species in the Balearic Sea (western Mediterranean). Prog. Oceanogr., 86, 21–38.
-
- Alldredge, A. (1976) Field behavior and adaptive strategies of appendicularians (Chordata: Tunicata). Mar. Biol., 38, 29–39.
-
- Arreguin-Sanchez, F., Zetina-Rejón, M., Manickchand-Heileman, S., Ramırez-Rodrıguez, M. and Vidal, L. (2004) Simulated response to harvesting strategies in an exploited ecosystem in the southwestern Gulf of Mexico. Ecol. Model., 172, 421–432.
-
- Bakun, A. (2006) Fronts and eddies as key structures in the habitat of marine fish larvae: opportunity, adaptive response and competitive advantage. Sci. Mar., 70, 105–122.
-
- Bakun, A. and Broad, K. (2003) Environmental ‘loopholes’ and fish population dynamics: comparative pattern recognition with focus on El Niño effects in the Pacific. Fish. Oceanogr., 12, 458–473.
LinkOut - more resources
Full Text Sources

