Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence
- PMID: 36046184
- PMCID: PMC9420854
- DOI: 10.3389/fcvm.2022.942971
Minor perturbations of thyroid homeostasis and major cardiovascular endpoints-Physiological mechanisms and clinical evidence
Abstract
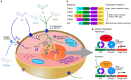
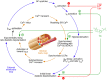
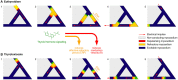
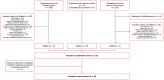
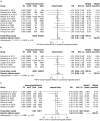
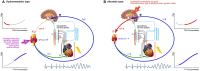
It is well established that thyroid dysfunction is linked to an increased risk of cardiovascular morbidity and mortality. The pleiotropic action of thyroid hormones strongly impacts the cardiovascular system and affects both the generation of the normal heart rhythm and arrhythmia. A meta-analysis of published evidence suggests a positive association of FT4 concentration with major adverse cardiovascular end points (MACE), but this association only partially extends to TSH. The risk for cardiovascular death is increased in both subclinical hypothyroidism and subclinical thyrotoxicosis. Several published studies found associations of TSH and FT4 concentrations, respectively, with major cardiovascular endpoints. Both reduced and elevated TSH concentrations predict the cardiovascular risk, and this association extends to TSH gradients within the reference range. Likewise, increased FT4 concentrations, but high-normal FT4 within its reference range as well, herald a poor outcome. These observations translate to a monotonic and sensitive effect of FT4 and a U-shaped relationship between TSH and cardiovascular risk. Up to now, the pathophysiological mechanism of this complex pattern of association is poorly understood. Integrating the available evidence suggests a dual etiology of elevated FT4 concentration, comprising both ensuing primary hypothyroidism and a raised set point of thyroid function, e. g. in the context of psychiatric disease, chronic stress and type 2 allostatic load. Addressing the association between thyroid homeostasis and cardiovascular diseases from a systems perspective could pave the way to new directions of research and a more personalized approach to the treatment of patients with cardiovascular risk.
Keywords: MACE; cardiac electrophysiology; hypothyroidism; sudden cardiac death; thyroid function; thyrotoxicosis; type 2 allostatic load; ventricular arrhythmia.
Copyright © 2022 Müller, Leow and Dietrich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

